142824
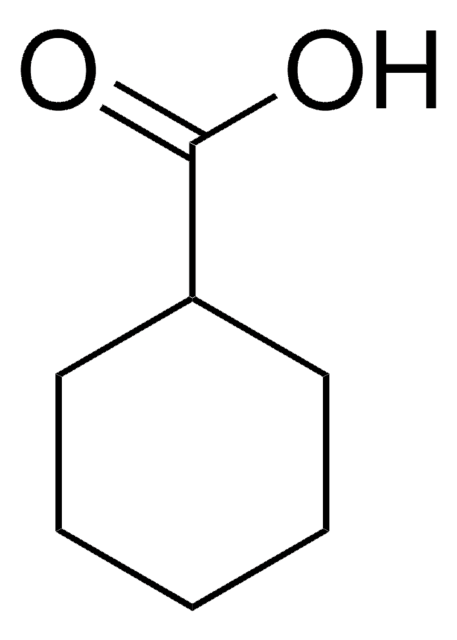
1-Methyl-1-cyclohexanecarboxylic acid
99%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
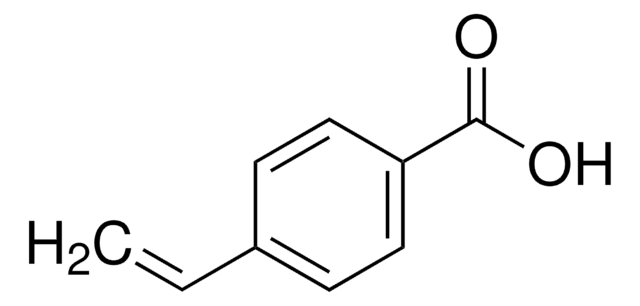
CH3C6H10CO2H
Número de CAS:
Peso molecular:
142.20
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Ensayo
99%
Formulario
solid
bp
234 °C (lit.)
mp
36-39 °C (lit.)
grupo funcional
carboxylic acid
cadena SMILES
CC1(CCCCC1)C(O)=O
InChI
1S/C8H14O2/c1-8(7(9)10)5-3-2-4-6-8/h2-6H2,1H3,(H,9,10)
Clave InChI
REHQLKUNRPCYEW-UHFFFAOYSA-N
Categorías relacionadas
Descripción general
1-Methyl-1-cyclohexanecarboxylic acid is the structural analog of valproic acid and its pharmacokinetic action has been studied in female Sprague-Dawley rats.
Aplicación
1-Methyl-1-cyclohexanecarboxylic acid was used as internal standard during the determination of valproic acid metabolites.
Acciones bioquímicas o fisiológicas
1-Methyl-1-cyclohexanecarboxylic acid is an anticonvulsant drug and causes maturation of murine neuroblastoma cells in vitro.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
213.8 °F - closed cup
Punto de inflamabilidad (°C)
101.00 °C - closed cup
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
J C Larcher et al.
Experimental cell research, 203(1), 72-79 (1992-11-01)
Adriamycin, an anticancer agent acting on topoisomerase II, promotes the arrest of cell division and neurite extension in a "neurite-minus" murine neuroblastoma cell line, N1A-103. This morphological differentiation is accompanied by a blockade in the S phase of the cell
J C Larcher et al.
Oncogene, 6(4), 633-638 (1991-04-01)
Using clones N1E-115 and N1A-103 from mouse neuroblastoma C1300, a comparative analysis of c- and N-myc gene expression was undertaken both in proliferating cells and in cultures exposed to conditions which induce differentiation. Under the latter conditions, while N1E-115 cells
A J Sadeque et al.
The Journal of pharmacology and experimental therapeutics, 283(2), 698-703 (1997-11-14)
Cytochrome P450-dependent desaturation of the anticonvulsant drug valproic acid (VPA) results in formation of the hepatotoxin, 4-ene-VPA. Polytherapy with other anticonvulsants which are known P450 inducers increases the flux through this bioactivation pathway. The aim of the present study was
S A Fischkoff et al.
Journal of biological response modifiers, 3(2), 132-137 (1984-01-01)
The anticonvulsant drug 1-methyl-1-cyclohexanecarboxylic acid ( MCCA ) has been shown to cause maturation of murine neuroblastoma cells in vitro at concentrations that are pharmacologically achievable. HL-60 human promyelocytic leukemia cells cultured with this drug underwent a dose-dependent decrease in
J L Vayssiere et al.
Biochemical and biophysical research communications, 140(3), 789-796 (1986-11-14)
CCA, a potent neuroblastoma differentiation inducer, was shown by oxygraphic measurements to reduce significantly the O2 consumption of whole neuroblastoma cells as of mitochondria purified from neuroblastoma or mouse cortex. The effect of CCA on the respiration was compared to
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico