U5507
Ubiquitin human
recombinant, expressed in E. coli (N-terminal histidine tagged)
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Recommended Products
biological source
human
Quality Level
recombinant
expressed in E. coli (N-terminal histidine tagged)
Assay
≥95% (GE)
form
lyophilized powder
mol wt
10.7 kDa
technique(s)
ligand binding assay: suitable
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
General description
Ubiquitin, a globular protein is made of 76 amino acids and has lysine residues on its surface. It has a molecular weight of 8565 Da.
Application
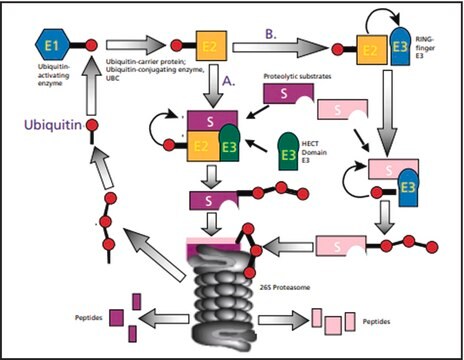
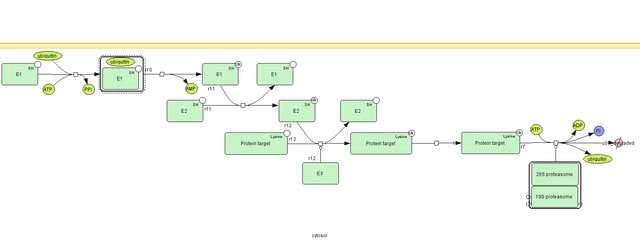
N-Terminal histidine-tagged ubiquitin can replace native ubiquitin in formation of poly-ubiquitin—protein conjugates. The histidine tag enables separation and enrichment of protein conjugates on a Ni(II) column and detection of conjugates in western blot by anti-histidine-tag antibodies.
Ubiquitin human can be used as a test compound for studying the isolation and characterization of rice E3-ubiquitin ligase and the role of OsHOS1 gene in the modulation of the cold stress response.
Ubiquitin human has been used:
- as a substrate in in vitro ubiquitination assays
- as substrate in ADP-ribosylation and ubiquitylation assays
- to supplement the 64 ng of endogenous ubiquitin in fresh blood for experiment grouping in order to study its potential clinical impact on cancer prognosis
Biochem/physiol Actions
Ubiquitin is a small regulatory protein present in eukaryote tissues. Exogenous ubiquitin can stimulate apoptosis in numerous cell lines. E7 protein of human papilloma virus-16 stimulates Retinoblastoma Protein degradation via Ubiquitin-Proteasome Pathway.
Ubiquitin plays a key role in normal eukaryotic cell function. It participates in the development and function of the immune system. This immunophilin is also known to participate in immune homeostasis. The ubiquitin peptide fragment (U50-59) is capable of blocking cellular and humoral immunity.
Preparation Note
Ubiquitin human can dissolved in 0.02 M Tris-HCl at a concentration of 1.00 - 1.10 mg/ml to yield a clear to slightly hazy, colorless solution.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
New crystal form of human ubiquitin in the presence of magnesium
Camara-Artigas A, et al.
Acta Crystallographica. Section F, Structural Biology Communications, 72(1), 29-35 (2016)
Extracellular Ubiquitin is the Causal Link between Stored Blood Transfusion Therapy and Tumor Progression in a Melanoma Mouse Model
Zhang J, et al.
Journal of Cancer, 10(12), 2822-2835 (2019)
Jingjun Zhang et al.
Journal of Cancer, 10(12), 2822-2835 (2019-07-02)
Background: The transfusion of blood that has been stored for some time was found to be associated with transfusion-related immune modulation (TRIM) responses in cancer patients, which could result in poor clinical outcomes, such as tumor recurrence, metastasis and reduced
Proteomics strategy to identify substrates of LNX, a PDZ domain-containing E3 ubiquitin ligase
Guo Z, et al.
Journal of Proteome Research, 11(10), 4847-4862 (2012)
Antigenic mimicry of ubiquitin by the gut bacterium Bacteroides fragilis: a potential link with autoimmune disease
Stewart L, et al.
Clinical and Experimental Immunology, 194(2), 153-165 (2018)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service