T7750
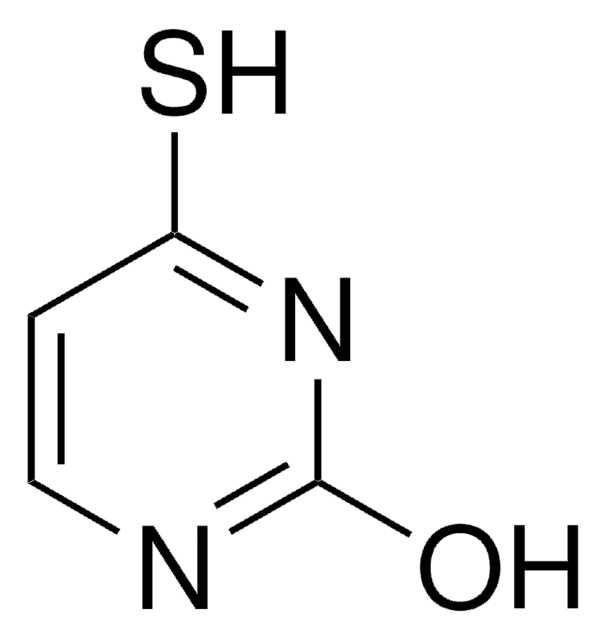
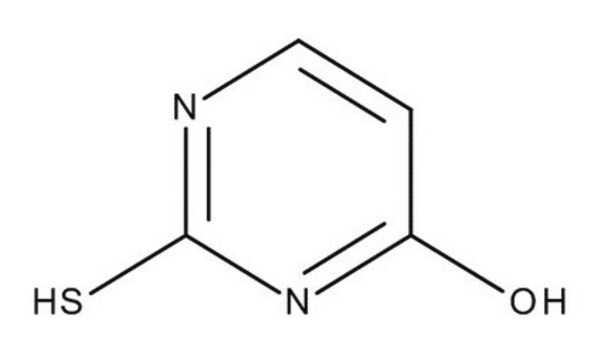
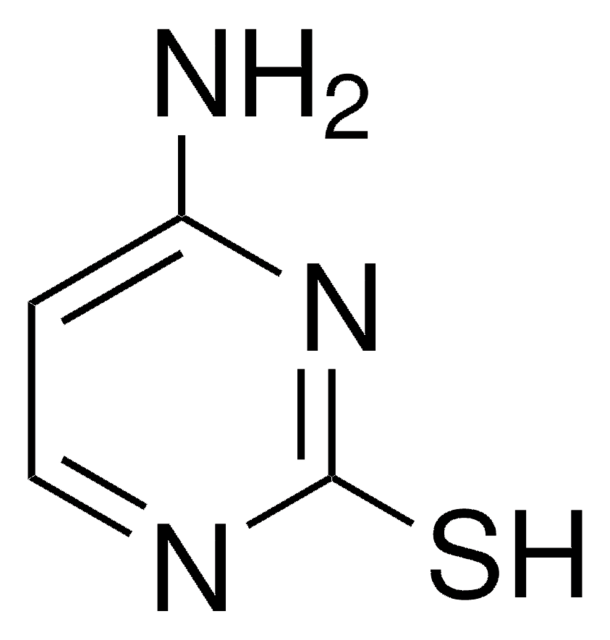
2-Thiouracil
≥99%
Synonym(s):
(2-thioxo -2, 3-dihydropyrimidin-4(1H)-one), 2TU, 4-Hydroxy-2-mercaptopyrimidine
Sign Into View Organizational & Contract Pricing
All Photos(5)
About This Item
Empirical Formula (Hill Notation):
C4H4N2OS
CAS Number:
Molecular Weight:
128.15
Beilstein:
112227
EC Number:
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.51
Recommended Products
biological source
synthetic
Quality Level
Assay
≥99%
form
powder
mp
>300 °C (lit.)
solubility
1 M NaOH: 5 mg/mL, clear to very slightly hazy
1 M NaOH: 50 mg/mL, colorless to faintly yellow
SMILES string
O=C1NC(=S)NC=C1
InChI
1S/C4H4N2OS/c7-3-1-2-5-4(8)6-3/h1-2H,(H2,5,6,7,8)
InChI key
ZEMGGZBWXRYJHK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Thiouracil is a thio-derivative of uracil, a pyrimidine nucleobase.This antithyroid drug has less solubility in H2O & organic solvents.
Application
2-Thiouracil may be used:
- in the synthesis and characterization of silver colloid and film substrates and their applications in surface-enhanced Raman scattering (SERS)
- to study its electro-oxidation and determination at titanium dioxide (TiO2) nanoparticles-modified gold electrode
- to study the effect of methylation on the deactivation mechanism or the triplet-state dynamics of 2-thiouracil using time-resolved photoelectron spectroscopy
Biochem/physiol Actions
2-Thiouracil acts as an anticancer, antithyroid, and antiviral agent. 2-Thiouracil is a selective melanoma-seeker and competitive inhibitor of neuronal nitric oxide synthase. It also forms complexes with transition metals such as gold (Au), chromium (Cr), zinc (Zn), and silver (Ag).2-thiouracil can be incorporated into the tissue culture medium to inhibit virus replication or movement from infected to healthy tissues during chemotherapy.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A Palumbo et al.
FEBS letters, 485(2-3), 109-112 (2000-11-30)
2-thiouracil (TU), an established antithyroid drug and melanoma-seeker, was found to selectively inhibit neuronal nitric oxide synthase (nNOS) in a competitive manner (K(i)=20 microM), being inactive on the other NOS isoforms. The drug apparently interfered with the substrate- and tetrahydrobiopterin
Silver colloid and film substrates in surface-enhanced Raman scattering for 2-thiouracil detection
Al-Shalalfeh MM, et al.
Royal Society of Chemistry Advances, 6, 75282-75292 (2016)
Karina Kraszewska et al.
Bioorganic & medicinal chemistry, 19(7), 2443-2449 (2011-03-15)
4-Pyrimidinone ribofuranoside (H(2)o(4)U) and 4-pyrimidinone 2'-deoxyribofuranoside (dH(2)o(4)U) were synthesized by the oxidative desulfurization of parent 2-thiouracil nucleosides with m-chloroperbenzoic acid. The crystal structures of H(2)o(4)U and dH(2)o(4)U and their conformations in solution were determined and compared with corresponding 2-thiouracil and
Hiroyuki Minami et al.
The Journal of prosthetic dentistry, 106(6), 378-385 (2011-12-03)
Although the effectiveness of primers for resin bonding to noble alloys has been demonstrated, no effective clinical technique for bonding to noble metal ceramic alloys has been established. The purpose of this study was to evaluate the effects of metal
K P Prasanthkumar et al.
The journal of physical chemistry. A, 116(44), 10712-10720 (2012-10-16)
The reaction of hydroxyl radical ((•)OH) with the nucleic acid base analogue 2-thiouracil (1) has been studied by pulse radiolysis experiments and DFT. The generic intermediate radicals feasible for the (•)OH reactions with 1, namely, one electron oxidation product (1(•+))
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service