T7194
Anti-phospho-Tau (pThr231) antibody produced in rabbit
affinity isolated antibody, buffered aqueous glycerol solution
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
biological source
rabbit
Quality Level
conjugate
unconjugated
antibody form
affinity isolated antibody
antibody product type
primary antibodies
clone
polyclonal
form
buffered aqueous glycerol solution
species reactivity
mouse, rat, human
technique(s)
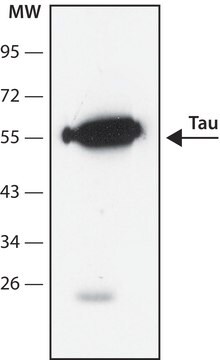
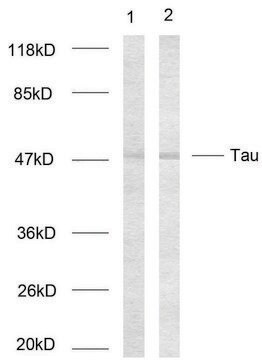
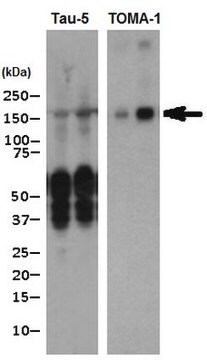
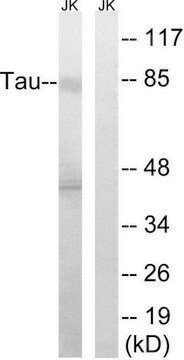
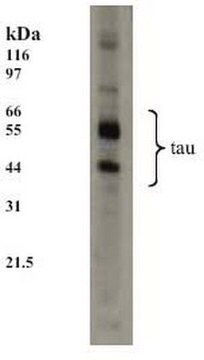
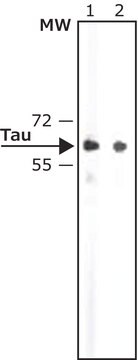
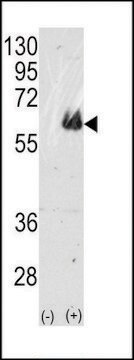
western blot: 1:1,000 using recombinant human tau treated with GSK-3β
UniProt accession no.
shipped in
wet ice
storage temp.
−20°C
target post-translational modification
phosphorylation (pThr231)
Gene Information
human ... MAPT(4137)
mouse ... Mapt(17762)
rat ... Mapt(29477)
Specificity
Peptide competition studies demonstrate the specificity of the antibody – only the phosphopeptide immunogen blocks the antibody signal.
Immunogen
chemically synthesized phosphopeptide derived from the region of human tau that contains threonine231.
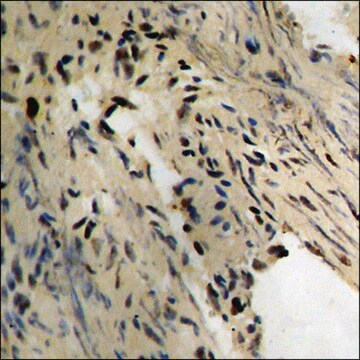
Application
Applications in which this antibody has been used successfully, and the associated peer-reviewed papers, are given below.
Western Blotting (1 paper)
Western Blotting (1 paper)
Physical form
Solution in Dulbecco′s phosphate buffered saline (without Mg2+ and Ca2+), pH 7.3, containing 50% glycerol, 1.0 mg/mL BSA (IgG, protease free) and 0.05% sodium azide.
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Su Y Yim et al.
International journal of molecular medicine, 24(1), 91-96 (2009-06-11)
Selenium reportedly contribute to the modulation process of protein phosphorylation to regulate various cellular functions including growth, differentiation, proliferation and development. The aim of this study was to investigate whether selenium and Selenoprotein M (SelM) affects the mechanism of Alzheimer's
Eun-Bum Kang et al.
Journal of exercise nutrition & biochemistry, 19(3), 199-209 (2015-11-04)
Neurofibrillary tangles, one of pathological features of Alzheimer's disease, are produced by the hyperphosphorylation and aggregation of tau protein. This study aimed to investigate the effects of treadmill exercise on PI3K/AKT/mTOR signal transmission, autophagy, and cognitive ability that are involved
Young Ju Lee et al.
Molecular medicine reports, 7(5), 1571-1578 (2013-04-03)
Alzheimer's disease (AD) is closely associated with significant defects in glucose metabolism. To investigate whether AD pathology induced by overexpression of human mutant presenilin 2 (PS2) protein induces changes in glucose metabolism, glucose‑related factors were analyzed in the brain of
S M Jenkins et al.
The Biochemical journal, 345 Pt 2, 263-270 (2000-01-06)
Tau is a microtubule-associated protein that is functionally modulated by phosphorylation and hyperphosphorylated in several neurodegenerative diseases. Because phosphorylation regulates both normal and pathological tau functioning, it is of great interest to identify the signalling pathways and enzymes capable of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service