F3680
10058-F4
≥98% (HPLC), solid
Synonym(s):
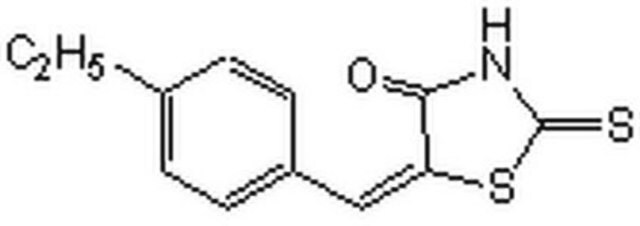
5-[(4-Ethylphenyl)methylene]-2-thioxo-4-thiazolidinone
About This Item
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
solid
color
yellow
solubility
DMSO: >10 mg/mL
H2O: <2 mg/mL
storage temp.
2-8°C
SMILES string
CCc1ccc(cc1)\C=C2\SC(=S)NC2=O
InChI
1S/C12H11NOS2/c1-2-8-3-5-9(6-4-8)7-10-11(14)13-12(15)16-10/h3-7H,2H2,1H3,(H,13,14,15)/b10-7+
InChI key
SVXDHPADAXBMFB-JXMROGBWSA-N
Application
- as c-Myc inhibitor to treat stromal cells
- as c-Myc inhibitor to determine the effect of c-Myc inhibition on cardiac progenitor cells (CPC) growth
- as c-Myc inhibitor to culture T cells
- to treat C4-2 cells to examine the activity of MST1 promoter luciferase reporter construct
Biochem/physiol Actions
Features and Benefits
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
We present an article about how proliferating cells require the biosynthesis of structural components for biomass production and for genomic replication.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service