F3503
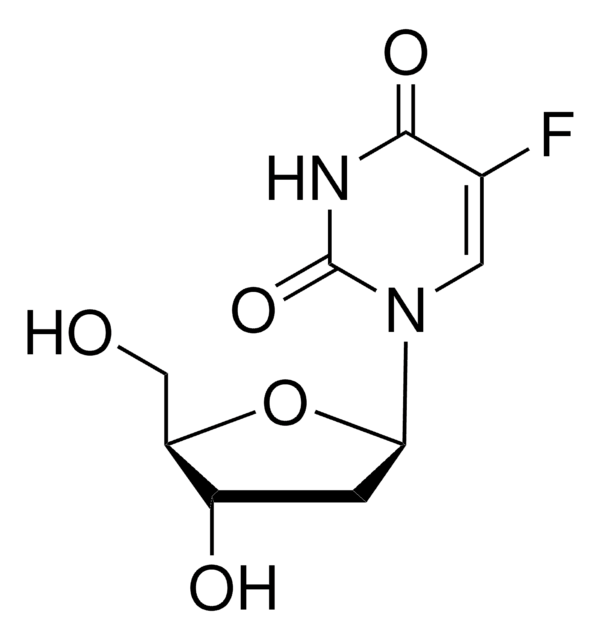
5-Fluoro-2′-deoxyuridine 5′-monophosphate sodium salt
~85%
Synonym(s):
FdUMP
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H12FN2O8P
CAS Number:
Molecular Weight:
326.17
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.51
Recommended Products
Assay
~85%
Quality Level
form
solid
storage temp.
−20°C
SMILES string
[Na].OC1CC(OC1COP(O)(O)=O)N2C=C(F)C(=O)NC2=O
InChI
1S/C9H12FN2O8P.Na.H/c10-4-2-12(9(15)11-8(4)14)7-1-5(13)6(20-7)3-19-21(16,17)18;;/h2,5-7,13H,1,3H2,(H,11,14,15)(H2,16,17,18);;
InChI key
DGEOOGBABXVZPJ-UHFFFAOYSA-N
General description
5- 5-fluoro-2′-deoxyuridylate (FdUMP) is an intracellularly generated metabolite of 5-Fluorouracil (5-FU), a widely used reagent in molecular biology to study DNA synthesis and metabolism.
Application
It has been used:
- as an anti-mitotic agent for culturing mouse primary cortical neurons.
- as a component of feeding media in the culturing of mouse primary cortical neurons.
- in studies on the activity and function of thymidylate synthase (TS).
Biochem/physiol Actions
5-Fluoro-2′-deoxyuridine 5′-monophosphate sodium salt (FdUMP) is an DNA synthesis inhibitor. FdUMP along with RNA synthesis inhibitor 3′-C-ethinylcytidine induces cell death in cervical cancer cells at nanomolar concentrations.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Induction of apoptosis in cervical cancer cells by the duplex drug 5-FdU--ECyd, coupling 2?-deoxy-5-fluorouridine and 3?-C-ethinylcytidine
Schott S and Bruning A
Gynecologic Oncology, 135(2), 342-348 (2014)
W H Gmeiner
Current medicinal chemistry, 12(2), 191-202 (2005-01-11)
Thymidylate synthase (TS) is a well-validated target for cancer chemotherapy. TS was established as the principal target of the widely used anticancer drug 5-fluorouracil (5FU). The 5FU metabolite FdUMP forms a covalent complex with TS that is stabilized by 5-formyl
Yoshihiro Nabeya et al.
Cancer science, 102(8), 1509-1515 (2011-05-13)
Thymidylate synthase (TS) plays a major role in the response to 5-fluorouracil (5-FU) by binding directly to the 5-FU metabolite, 5-fluoro-dUMP (FdUMP). The change in the TS expression levels after 5-FU administration was examined in parallel to 5-FU responsiveness in
Paul J Sapienza et al.
Biochemistry, 58(30), 3302-3313 (2019-07-10)
Thymidylate synthase (TS) is a dimeric enzyme conserved in all life forms that exhibits the allosteric feature of half-the-sites activity. Neither the reason for nor the mechanism of this phenomenon is understood. We used a combined nuclear magnetic resonance (NMR)
Manee Chanama et al.
Molecular biology reports, 38(2), 1029-1037 (2010-06-26)
Thymidylate synthase (TS) of Plasmodium dihydrofolate reductase-thymidylate synthase (DHFR-TS) functions as a homodimeric enzyme with two active sites located near the subunit interface. The dimerization is essential for catalysis, since the active site of each subunit contains amino acid residues
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service