E8145
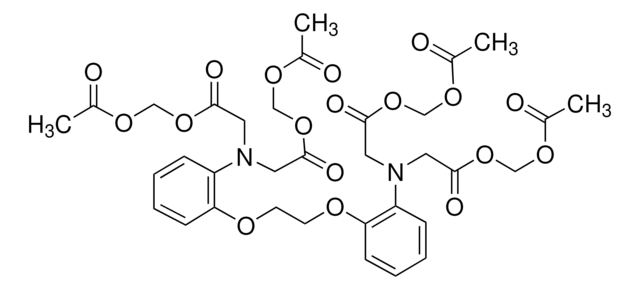
Ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid tetrasodium salt
≥97%
Synonym(s):
EGTA tetrasodium, Egtazic acid tetrasodium, Ethylene-bis(oxyethylenenitrilo)tetraacetic acid tetrasodium
About This Item
Recommended Products
Assay
≥97%
technique(s)
RNA purification: suitable
solubility
H2O: soluble 10%, clear, colorless
application(s)
life science and biopharma
SMILES string
[Na+].[Na+].[Na+].[Na+].[O-]C(=O)CN(CCOCCOCCN(CC([O-])=O)CC([O-])=O)CC([O-])=O
InChI
1S/C14H24N2O10.4Na/c17-11(18)7-15(8-12(19)20)1-3-25-5-6-26-4-2-16(9-13(21)22)10-14(23)24;;;;/h1-10H2,(H,17,18)(H,19,20)(H,21,22)(H,23,24);;;;/q;4*+1/p-4
InChI key
BAOGCDPNNOBWQZ-UHFFFAOYSA-J
Looking for similar products? Visit Product Comparison Guide
Application
- in media for chelating Ca2+ to facilitate efficient incorporation of Sr2+ into the oocytes
- as a component of phosphatase wash buffer used to wash magnetic beads during dephosphorylation and radiolabeling of RNA segments cross-linked to immunoprecipitated proteins
- as a component of oocyte activation media
Biochem/physiol Actions
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service