185698
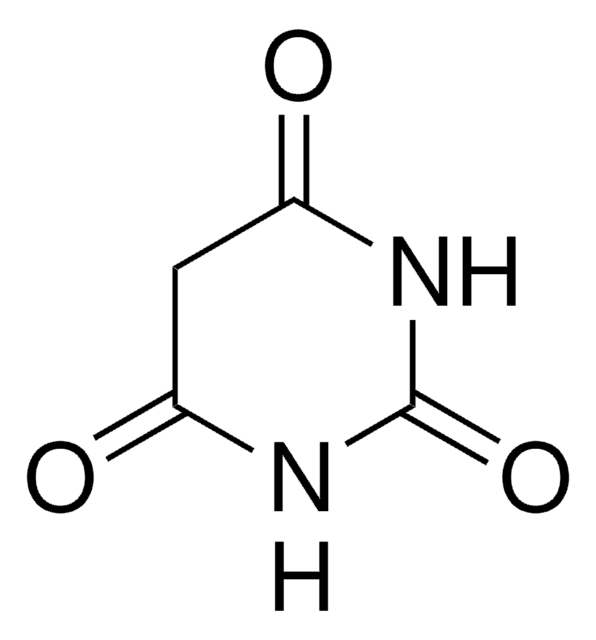
Barbituric acid
ReagentPlus®, 99%
Synonym(s):
2,4,6-Trihydroxypyrimidine, Malonylurea
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C4H4N2O3
CAS Number:
Molecular Weight:
128.09
Beilstein:
120502
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
99%
form
powder
mp
248-252 °C (dec.) (lit.)
SMILES string
O=C1CC(=O)NC(=O)N1
InChI
1S/C4H4N2O3/c7-2-1-3(8)6-4(9)5-2/h1H2,(H2,5,6,7,8,9)
InChI key
HNYOPLTXPVRDBG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Barbituric acid is a useful acid for organic and drug syntheses. Its dihydrate form can be synthesized from barbituric acid via crystallization from aqueous solution. Crystal structure of barbituric acid (in tautomeric form) has been investigated by a three dimensional fourier transform method. Its enol crystal form has been reported to be thermodynamically stable.
Application
Barbituric acid (BA) may be used in the preparation of the corresponding hemiaminals, via chemoselective reduction in the presence of SmI2/H2O reagent. It may be used in the preparation of BA- modified conjugated carbon nitride nanosheets.
It may be used to synthesize:
- 5-ylidenebarbituric acid derivatives via Knoevenagel condensation with aromatic and α,β-conjugated aromatic aldehydes
- 5-diaminomethylenebarbiturates by reacting with substituted carbodiimides
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
302.0 °F - closed cup
Flash Point(C)
150.00 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Selective reduction of barbituric acids using SmI2/H2O: synthesis, reactivity, and structural analysis of tetrahedral adducts.
Michal Szostak et al.
Angewandte Chemie (International ed. in English), 52(48), 12559-12563 (2013-10-15)
A simple method for knoevenagel condensation of a, ?-conjugated and aromatic aldehydes with barbituric acid.
Jursic BS.
Journal of Heterocyclic Chemistry, 38(3), 655-657 (2001)
Photocatalytic reduction of CO2 by graphitic carbon nitride polymers derived from urea and barbituric acid.
Qin J, et al.
Applied Catalysis. B, Environmental, 179, 1-8 (2015)
The crystal structure of anhydrous barbituric acid.
Bolton W.
Acta Crystallographica, 16(3), 166-173 (1963)
Preparation of 5-diaminomethylenebarbiturates by barbituric acid addition to carbodiimides.
Jursic BS et al.
Tetrahedron, 59(19), 3427-3432 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service