03143422001
Roche
Genopure Plasmid Maxi Kit
kit of for 10 isolations from 30 to 150 ml
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
UNSPSC Code:
41105500
NACRES:
NA.55
Recommended Products
General description
For large-scale (maxi) preparation of plasmid DNA.
Application
The Genopure Plasmid Maxi Kit prepares transfection-grade plasmid DNA in large quantities (up to 500 μg plasmid) from bacterial cultures. Isolated plasmid is suitable for most molecular biology applications:
- Transfection
- Southern blotting
- Sequencing
- PCR/long PCR
- Restriction digestion
- Cloning
Features and Benefits
The Genopure Plasmid Maxi Kit prepares highly purified plasmid DNA in large quantities using a modified alkaline lysis method.
- Save time with ready-to-use reagents.
- Purify all sizes and types of plasmid
- Process multiple samples in parallel
- Eliminate the use of hazardous organic compounds
- Obtain higher purity plasmid DNA
Components
- Suspension Buffer
- RNase A
- Lysis Buffer
- Neutralization Buffer
- Equilibration Buffer
- Wash Buffer
- Elution Buffer
- NucleoBond AX 500 Columns
- Folded Filters (240 mm diameter)
- Sealing Rings
Quality
Plasmid DNA purified by this kit has been tested for restriction digestion; pUC 19 was isolated from transformed HB101 as described in the protocol. 1 μg of plasmid was completely digested with 1 U Msp I for 2 hours at +37°C, as shown by agarose gel analysis.
Plasmid recovery was tested with 250 μg purified plasmid. The recovery was >90%, with more than 80% in supercoiled form. The yield of plasmid DNA was determined by isolating pBS from DH5a cells. From 150 ml culture volume with a density of A600 between 3 and 6, >400 μg of plasmid DNA was obtained.
The purity (checked by the ratio of A260/A280) is 1.8 + 0.2.
RNA contamination was analyzed with 3 μg pBS purified with the standard procedure and checked by electrophoresis on an agarose gel. No RNA was detected.
The kit components have been tested for the absence of nucleases according to current quality control procedures.
Plasmid recovery was tested with 250 μg purified plasmid. The recovery was >90%, with more than 80% in supercoiled form. The yield of plasmid DNA was determined by isolating pBS from DH5a cells. From 150 ml culture volume with a density of A600 between 3 and 6, >400 μg of plasmid DNA was obtained.
The purity (checked by the ratio of A260/A280) is 1.8 + 0.2.
RNA contamination was analyzed with 3 μg pBS purified with the standard procedure and checked by electrophoresis on an agarose gel. No RNA was detected.
The kit components have been tested for the absence of nucleases according to current quality control procedures.
Preparation Note
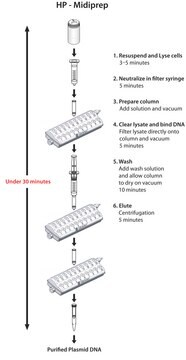
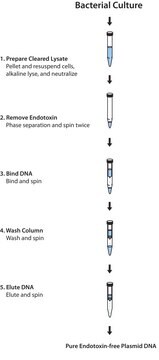
The isolation procedure is based on a modified alkaline lysis protocol and can be divided into the following steps:The bacteria are partially lysed, allowing the plasmid DNA to escape the cell wall into the supernatant. The larger E. coli chromosomal DNA is trapped in the cell wall. The lysate is cleared of cellular debris by filtration or centrifugation, and the plasmid DNA-containing fraction is loaded onto a pre-equilibrated column. Since the cleared lysate is applied to the column in a low-salt buffer, plasmid DNA binds to the macroporous anion-exchange material in the column. Cellular impurities are eluted from the column with a high-salt wash. Finally, the plasmid DNA is eluted from the column. Recovered DNA is precipitated from the eluate to remove salt and concentrate the plasmid.
Analysis Note
Sample:
E. coli culture that contains a high-copy number plasmid: 30 to 150 mL bacterial culture
E. coli culture that contains a low-copy number plasmid: 100 to 500 mL bacterial culture
Plasmid Size: The isolation procedure is suitable for all sizes of plasmid. Note: Lysates of larger constructs (up to 100 kb) should be cleared by filtration rather than centrifugation to avoid shearing the plasmid.
Time Required: 75 minutes (including filtration of the lysate)
Typical Yield:
High-copy number plasmid: 3 to 5 μg/mL culture
Low-copy number plasmid: 0.2 to 1 μg/mL culture
Product Purity: Isolated plasmid DNA is free of other bacterial components, including RNA.
E. coli culture that contains a high-copy number plasmid: 30 to 150 mL bacterial culture
E. coli culture that contains a low-copy number plasmid: 100 to 500 mL bacterial culture
Plasmid Size: The isolation procedure is suitable for all sizes of plasmid. Note: Lysates of larger constructs (up to 100 kb) should be cleared by filtration rather than centrifugation to avoid shearing the plasmid.
Time Required: 75 minutes (including filtration of the lysate)
Typical Yield:
High-copy number plasmid: 3 to 5 μg/mL culture
Low-copy number plasmid: 0.2 to 1 μg/mL culture
Product Purity: Isolated plasmid DNA is free of other bacterial components, including RNA.
Other Notes
For life science research only. Not for use in diagnostic procedures.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 3 - Met. Corr. 1 - Skin Corr. 1
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
100.4 °F
Flash Point(C)
38 °C
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Sharon Yunger et al.
Nature protocols, 8(2), 393-408 (2013-02-22)
Transcription kinetics of actively transcribing genes in vivo have generally been measured using tandem gene arrays. However, tandem arrays do not reflect the endogenous state of genome organization in which genes appear as single alleles. Here we present a robust
Vivian I Bonano et al.
Nature protocols, 2(9), 2166-2181 (2007-09-15)
Imaging technologies are influencing the way we study regulatory processes in vivo. Several recent reports use fluorescence minigenes to image alternative splicing events in living cells and animals. This type of reporter is being used to generate transgenic mice to
Haruka Sato et al.
eLife, 11 (2022-03-16)
Neuronal abundance and thickness of each cortical layer are specific to each area, but how this fundamental feature arises during development remains poorly understood. While some of area-specific features are controlled by intrinsic cues such as morphogens and transcription factors
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service