PCYTMG-40K-PX23
MILLIPLEX® Non-Human Primate Cytokine Magnetic Bead Panel - Premixed 23 Plex - Immunology Multiplex Assay
Simultaneously analyze multiple cytokine and chemokine biomarkers with Bead-Based Multiplex Assays using the Luminex technology in non-human primate serum, plasma and cell culture samples.
Synonym(s):
Luminex® non-human primate cytokine immunoassay, Millipore non-human primate cytokine panel, Monkey cytokine multiplex kit
About This Item
Recommended Products
Quality Level
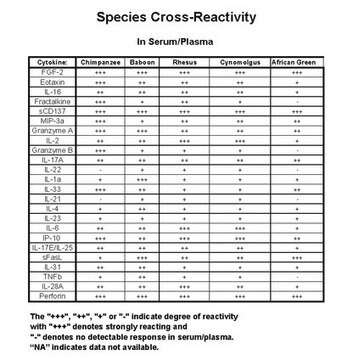
species reactivity
nonhuman primates
manufacturer/tradename
Milliplex®
assay range
accuracy: 70-101%
linearity: 110.3%
(1:06)
linearity: 121.6%
(1:12)
linearity: 126.2%
(1:24)
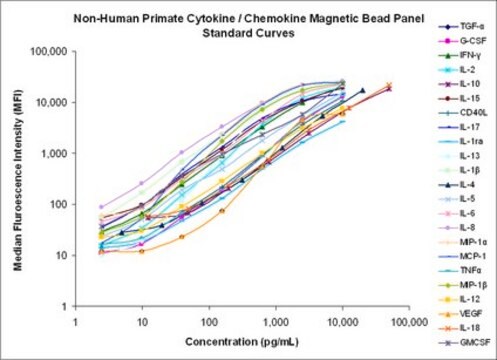
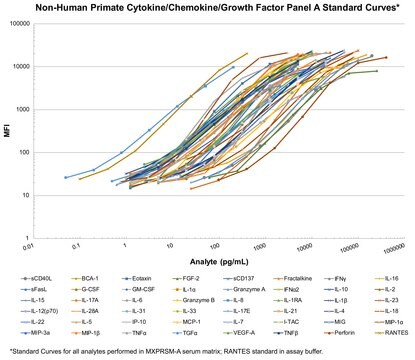
standard curve range: 12.2-50,000 pg/mL
(IL-10, IL-18)
standard curve range: 2.4-10,000 pg/mL
standard curve range: 4.9-20,000 pg/mL
(IL-4)
technique(s)
multiplexing: suitable
compatibility
configured for Premixed
detection method
fluorometric (Luminex xMAP)
shipped in
wet ice
General description
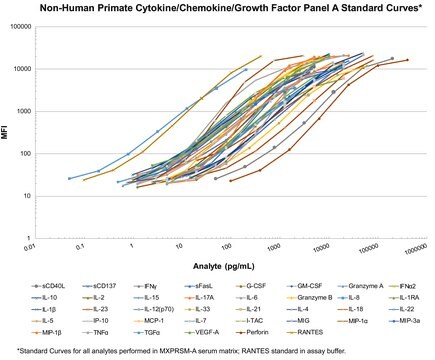
The MILLIPLEX® Non-Human Primate Cytokine panel is to be used for the simultaneous quantification of any or all of the following in tissue/cell lysate and culture supernatant
samples and serum or plasma samples: G-CSF, GM-CSF, IFNγ, IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL8, IL-10, IL-12/23(p40), IL-13, IL-15, IL-17A, MCP-1, MIP-1β, MIP-1α, sD40L, TGF-α, TNF-α, VEGF, and IL-18.
The Luminex® xMAP® platform uses a magnetic bead immunoassay format for ideal speed and sensitivity to quantitate multiple analytes simultaneously, dramatically improving productivity while conserving valuable sample volume.
Panel Type: Cytokines/Chemokines
Specificity
The antibody pairs in the panel are specific only to the desired analyte and exhibit no or negligible cross-reactivity with other analytes in the panel
Application
- Analytes: G-CSF, GM-CSF, IFN-γ, IL-1Ra, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12/23 (p40), IL-13, IL-15, IL-17A, IL-18, MCP-1, MIP-1α, MIP-1β, sCD40L, TGF-α, TNF-α, VEGF-A
- Recommended Sample type: Serum, plasma, and cell culture supernatants
- Recommended Sample dilution: Neat
- Assay Run Time: Overnight or one day. For best results, an overnight incubation is recommended
- Research Category: Inflammation & Immunology
Packaging
Storage and Stability
Other Notes
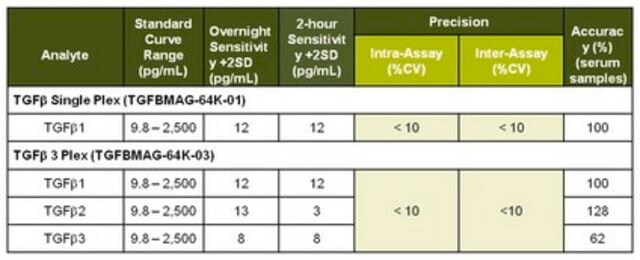
Standard Curve Range: Refer to QC analysis sheet for exact concentration (sCD40L, TNFα)
Legal Information
Disclaimer
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service