MAB858-2-5
Anti-RSV Antibody, glycoprotein, all type A, B strains, clone 131-2G
clone 131-2G, Chemicon®, from mouse
Synonym(s):
RSV
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352203
eCl@ss:
32160702
NACRES:
NA.41
Recommended Products
biological source
mouse
Quality Level
antibody form
purified immunoglobulin
antibody product type
primary antibodies
clone
131-2G, monoclonal
species reactivity
human
manufacturer/tradename
Chemicon®
technique(s)
ELISA: suitable
immunofluorescence: suitable
isotype
IgG1κ
shipped in
wet ice
General description
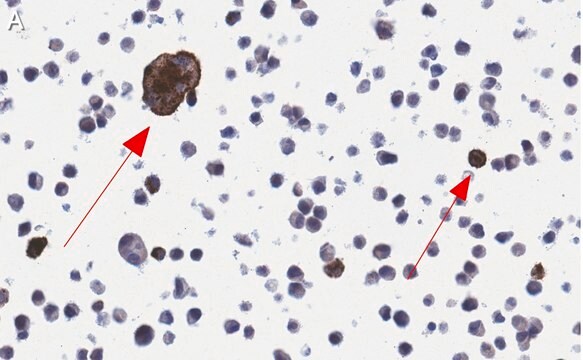
RSV is a labile paramyxovirus that produces a characteristic fusion of human cells in tissue culture--the syncytial effect. Two subtypes, A and B, have been identified. Subtype B are characterized as the asymptomatic strains of the virus. The more severe clinical illnesses involve Subtype A strains.
Specificity
Recognizes Glycoprotein, 89 kD. Recognizes both A and B strains.
Immunogen
A2
Application
Anti-RSV Antibody, glycoprotein, all type A, B strains, clone 131-2G detects level of Respiratory Syncytial Virus & has been published & validated for use in ELISA & IF.
ELISA
Indirect Immunofluorescence (fresh frozen tissue sections)
Final working dilutions must be determined by end user.
Indirect Immunofluorescence (fresh frozen tissue sections)
Final working dilutions must be determined by end user.
Research Category
Infectious Diseases
Infectious Diseases
Research Sub Category
Infectious Diseases - Viral
Infectious Diseases - Viral
Physical form
Format: Purified
Protein A Purified mouse immunoglobulin in 20 mM sodium phosphate, 250 mM NaCl, pH. 7.6, with 0.1% sodium azide as a preservative.
Protein A purified
Storage and Stability
Maintain at 2 to 8° C in aliquots for up to 12 months after date of receipt. Do not store in diluted format.
Analysis Note
Control
RSV Control Slides, Catalogue Number 5012-5
RSV Control Slides, Catalogue Number 5012-5
Other Notes
Concentration: Please refer to the Certificate of Analysis for the lot-specific concentration.
Legal Information
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Christopher C Stobart et al.
Nature communications, 7, 13916-13916 (2016-12-22)
Respiratory syncytial virus (RSV) is a leading cause of infant hospitalization and there remains no pediatric vaccine. RSV live-attenuated vaccines (LAVs) have a history of safe testing in infants; however, achieving an effective balance of attenuation and immunogenicity has proven
Debby Kruijsen et al.
Journal of immunology (Baltimore, Md. : 1950), 185(11), 6489-6498 (2010-10-26)
Following infection with respiratory syncytial virus (RSV), reinfection in healthy individuals is common and presumably due to ineffective memory T cell responses. In peripheral blood of healthy adults, a higher CD4(+)/CD8(+) memory T cell ratio was observed compared with the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service