W398802
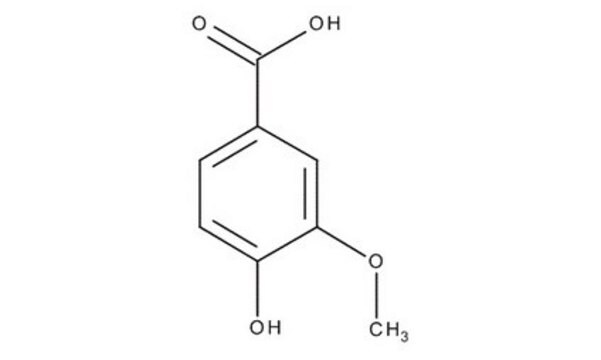
Vanillic acid
≥97%, FG
Synonym(s):
4-Hydroxy-3-methoxybenzoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOC6H3(OCH3)CO2H
CAS Number:
Molecular Weight:
168.15
FEMA Number:
3988
Beilstein:
2208364
EC Number:
MDL number:
UNSPSC Code:
12164502
PubChem Substance ID:
Flavis number:
8.043
NACRES:
NA.21
Recommended Products
biological source
synthetic
Quality Level
grade
FG
Halal
Kosher
reg. compliance
EU Regulation 1334/2008 & 178/2002
Assay
≥97%
mp
208-210 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
Organoleptic
creamy; milk; sweet; vanilla
SMILES string
COc1cc(ccc1O)C(O)=O
InChI
1S/C8H8O4/c1-12-7-4-5(8(10)11)2-3-6(7)9/h2-4,9H,1H3,(H,10,11)
InChI key
WKOLLVMJNQIZCI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Natural occurrence: Guava, grape, brandy, rum, whiskey, sherry, red and white wines, Scotch and Canadian whiskey.
Vanillic acid is one of the key aromatic volatile compounds of vanilla beans.
Application
Vanillic acid is a phenolic derivative, which is generally used as a flavoring agent in food products. It can be used in the synthesis of a well-known flavoring agent vanillin.
Biochem/physiol Actions
Odor at 5%
Taste at 100 ppm
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Solid-liquid phase equilibrium and thermodynamic properties of vanillic acid in different pure solvents
Noubigh A and Abderrabba M
Journal of Molecular Liquids, 223, 261-266 (2016)
Vanillin and related flavor compounds in vanilla extracts made from beans of various global origins
Ranadive AS.
Journal of Agricultural and Food Chemistry, 40(10), 1922-1924 (1992)
Delphine Lamoral-Theys et al.
Bioorganic & medicinal chemistry, 18(11), 3823-3833 (2010-05-15)
A series of 33 novel divanillates and trivanillates were synthesized and found to possess promising cytostatic rather than cytotoxic properties. Several compounds under study decreased by >50% the activity of Aurora A, B, and C, and WEE1 kinase activity at
B L Lee et al.
Clinical chemistry, 39(9), 1788-1792 (1993-09-01)
We describe a sensitive and specific high-performance liquid-chromatographic method for determining the benzene metabolite, trans,trans-muconic acid (ttMA) in urine by measuring ultraviolet absorbance at 265 nm. We mix 1 mL of urine sample with 2 mL of Tris buffer containing
F Mashige et al.
Journal of chromatography. B, Biomedical applications, 658(1), 63-68 (1994-08-05)
An HPLC system for the simultaneous determination of acidic catecholamine metabolites, related compounds and 5-hydroxyindoleacetic acid (5-HIAA) in human urine was developed. A mixed-mode (C18/anion-exchange) column with isocratic elution using citrate buffer and an eight-channel electrochemical detector were used. Vanilmandelic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service