W291102
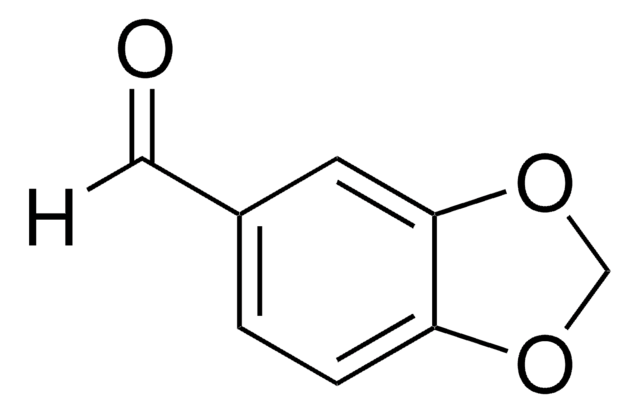
Piperonal
≥99%, FCC, FG
Synonym(s):
1,3-Benzodioxole-5-carboxaldehyde, 3,4-(Methylenedioxy)benzaldehyde, Heliotropin
About This Item
Recommended Products
biological source
synthetic
Quality Level
grade
FG
Fragrance grade
Halal
Kosher
Agency
follows IFRA guidelines
meets purity specifications of JECFA
reg. compliance
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FCC
FDA 21 CFR 182.60
vapor pressure
1 mmHg ( 87 °C)
Assay
≥99%
bp
264 °C (lit.)
mp
35-39 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
fragrance allergen
heliotropine
Organoleptic
cherry; sweet; vanilla
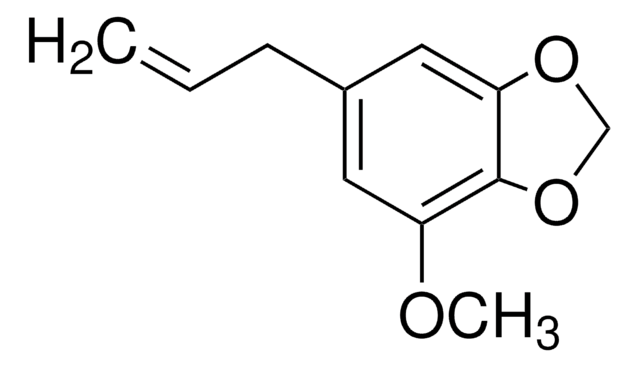
SMILES string
[H]C(=O)c1ccc2OCOc2c1
InChI
1S/C8H6O3/c9-4-6-1-2-7-8(3-6)11-5-10-7/h1-4H,5H2
InChI key
SATCULPHIDQDRE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- The synthesis and characterisation of MDMA derived from a catalytic oxidation of material isolated from black pepper reveals potential route specific impurities.: This study explores the synthesis and characterization of MDMA from piperonal, highlighting potential impurities unique to this synthesis route. This research has implications for forensic science and the identification of synthetic routes for MDMA (Plummer et al., 2016).
- Design, synthesis, and biological evaluation of platensimycin analogues with varying degrees of molecular complexity.: This paper details the synthesis of platensimycin analogues using piperonal derivatives. The study evaluates the biological activities of these analogues, contributing to the development of new antibacterial agents (Nicolaou et al., 2008).
- Synthesis and use of 4-peptidylhydrazido-N-hexyl-1,8-naphthalimides as fluorogenic histochemical substrates for dipeptidyl peptidase IV and tripeptidyl peptidase I.: This research presents the synthesis of piperonal-based substrates for histochemical applications, enabling the study of enzyme activities in biochemical assays (Ivanov et al., 2009).
Biochem/physiol Actions
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
150.1 °F
Flash Point(C)
65.62 °C
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service