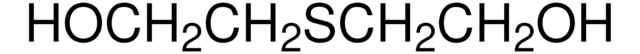T30007
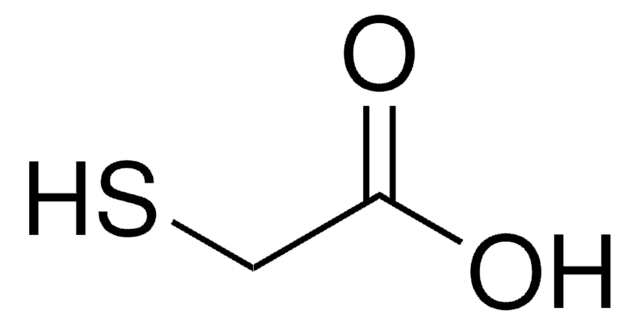
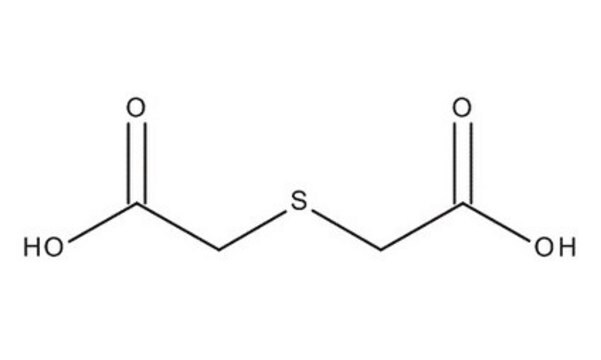
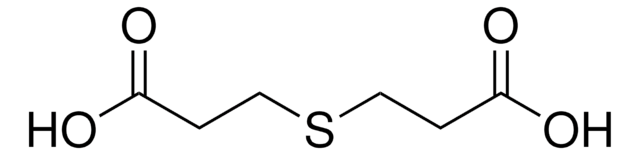
2,2′-Thiodiacetic acid
98%
Synonym(s):
2,2′-Thio-bis(acetic acid), Dicarboxydimethyl sulfide, Thiodiglycolic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
S(CH2COOH)2
CAS Number:
Molecular Weight:
150.15
Beilstein:
1764392
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
128-131 °C (lit.)
SMILES string
OC(=O)CSCC(O)=O
InChI
1S/C4H6O4S/c5-3(6)1-9-2-4(7)8/h1-2H2,(H,5,6)(H,7,8)
InChI key
UVZICZIVKIMRNE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
<ul>
<li><strong>Environmentally Safe Herbicides:</strong> It is used in the synthesis of quaternary ammonium mono- and bis-salts. This compound has been formulated into ammonium 2,2-thiodiacetates, serving as selective and environmentally safe herbicides. Its application underscores its utility in sustainable agriculture and safety in environmental management (Balczewski et al., 2018).</li>
</ul>
<li><strong>Environmentally Safe Herbicides:</strong> It is used in the synthesis of quaternary ammonium mono- and bis-salts. This compound has been formulated into ammonium 2,2-thiodiacetates, serving as selective and environmentally safe herbicides. Its application underscores its utility in sustainable agriculture and safety in environmental management (Balczewski et al., 2018).</li>
</ul>
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Isotachophoresis.
L Krivánková et al.
Methods in enzymology, 270, 375-401 (1996-01-01)
E B Tikhonova et al.
Mikrobiologiia, 71(2), 247-253 (2002-05-25)
The Alcaligenes xylosoxydans subsp. denitrificans strain TD1 capable of degrading thiodiglycol (TDG), a breakdown product of mustard gas, was isolated from soil contaminated with breakdown products of this chemical warfare agent. The selected stable variant of TD1 (strain TD2) can
J P Payan et al.
Journal of applied toxicology : JAT, 13(6), 417-422 (1993-11-01)
1,2-dichloroethane (DCE) is extensively metabolized and partially excreted in urine as thioether compounds, which include thiodiglycolic acid (TDGA). In this study, we have compared the urinary excretion of TDGA and thioethers in the rat after administration of increasing doses of
T Lee et al.
Biotechnology progress, 16(3), 363-367 (2000-06-03)
A Gram-negative bacterium, Alcaligenes xylosoxydans ssp. xylosoxydans (SH91), consumed thiodiglycol (TDG), the nontoxic hydrolysis product of sulfur mustard, as a primary carbon source and transformed TDG to commercially relevant chemical precursors, [(2-hydroxyethyl)thio]acetic acid (HETA) and thiodiglycolic acid (TDGA). Aerobic fed
T M Visarius et al.
Drug metabolism and disposition: the biological fate of chemicals, 26(3), 193-196 (1998-04-04)
Thiodiglycolic acid has been identified as a major metabolite of the anticancer drug ifosfamide in humans. Patients treated with 12-16 g ifosfamide/m2.day excreted thiodiglycolic acid ranging from 0.10 +/- 0.02 mmol on the first day of therapy, to a maximum
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service