I18008
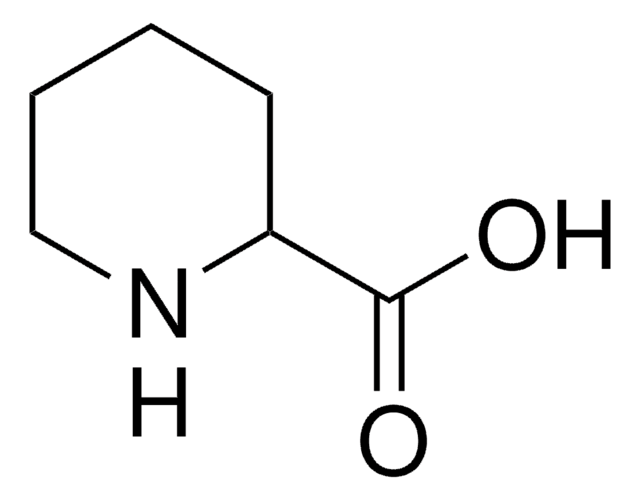
Isonipecotic acid
97%
Synonym(s):
4-Piperidinecarboxylic acid, Hexahydroisonicotinic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C6H11NO2
CAS Number:
Molecular Weight:
129.16
Beilstein:
112553
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
>300 °C (lit.)
SMILES string
OC(=O)C1CCNCC1
InChI
1S/C6H11NO2/c8-6(9)5-1-3-7-4-2-5/h5,7H,1-4H2,(H,8,9)
InChI key
SRJOCJYGOFTFLH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reactant for synthesis of:
- Antibiotic nitroxoline derivatives for cathepsin B inhibition
- Sphingosine-1-phosphate receptor agonists
- RhoA inhibitors for cardiovascular disease therapy
- Alkyl piperidine and piperazine hydroxamic acids as HDAC inhibitors
- CHK1 inhibitors
- IKK2 inhibitors for investigations into rheumatoid arthritis treatment
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Alicja Nowaczyk et al.
Journal of molecular graphics & modelling, 85, 171-181 (2018-09-17)
Inhibition of 4-aminobutanoic acid (GABA) uptake is a strategy for enhancing GABA transmission. The utility of this approach is demonstrated by the successful development of such agents for the treatment of epilepsy and pain. Existing reports on acute brain slice
Michela Semeraro et al.
Clinica chimica acta; international journal of clinical chemistry, 440, 108-112 (2014-12-03)
Pipecolic acid (PA) is an important biochemical marker for the diagnosis of peroxisomal disorders. PA is also a factor responsible for hepatic encephalopathy and a possible biomarker for pyridoxine-dependent seizures. We developed an easy and rapid PA quantification method, by
Iris Thondorf et al.
Bioorganic & medicinal chemistry, 19(21), 6409-6418 (2011-10-01)
The proton-coupled amino acid transporter hPAT1 has recently gained much interest due to its ability to transport small drugs thereby allowing their oral administration. A three-dimensional quantitative structure-activity relationship (3D QSAR) study has been performed on its natural and synthetic
Jing Yuan et al.
Nature chemical biology, 5(10), 765-771 (2009-09-08)
Studies of gene function and molecular mechanisms in Plasmodium falciparum are hampered by difficulties in characterizing and measuring phenotypic differences between individual parasites. We screened seven parasite lines for differences in responses to 1,279 bioactive chemicals. Hundreds of compounds were
Phedias Diamandis et al.
Nature chemical biology, 3(5), 268-273 (2007-04-10)
The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain cancer. However, the complete repertoire of signaling pathways that governs the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service