I10201
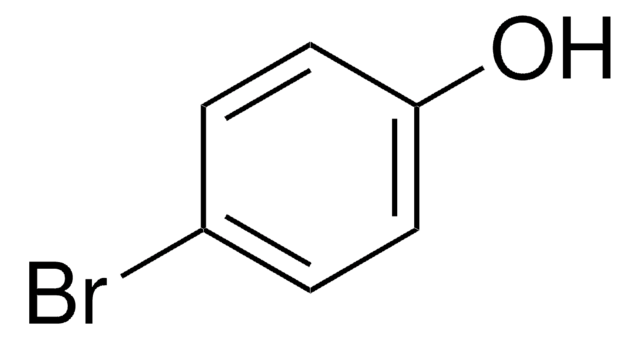
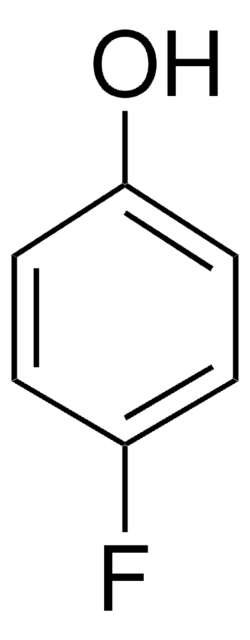
4-Iodophenol
99%
Synonym(s):
1-Iodo-4-hydroxybenzene, 4-Hydroxyiodobenzene, 4-Hydroxyphenyl iodide, p-Hydroxyiodobenzene, p-Iodophenol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
IC6H4OH
CAS Number:
Molecular Weight:
220.01
Beilstein:
1904544
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
bp
138 °C/5 mmHg (lit.)
mp
92-94 °C (lit.)
SMILES string
Oc1ccc(I)cc1
InChI
1S/C6H5IO/c7-5-1-3-6(8)4-2-5/h1-4,8H
InChI key
VSMDINRNYYEDRN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
4-Iodophenol can be used as a building block for the synthesis of:
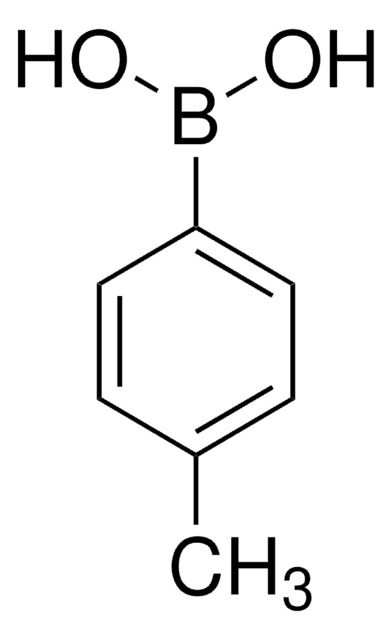
- Hydroxybiaryls by reacting with aryl boronic acids via Pd-catalyzed Suzuki-Miyaura coupling reaction.
- Aryl substituted olefins by reacting with acrylates via Pd-catalyzed Heck reaction.
- Iodinated-4-aryloxymethylcoumarins
- Hydroxylated stilbenoids and psammaplysenes A and B.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis, structure-activity relationship of iodinated-4-aryloxymethyl-coumarins as potential anti-cancer and anti-mycobacterial agents
Basanagouda, M, et al.
European Journal of Medicinal Chemistry, 74(1), 225-233 (2014)
Carbon- carbon coupling reactions catalyzed by heterogeneous palladium catalysts
Yin L and Liebscher J
Chemical Reviews, 107(1), 133-173 (2007)
Pd/C as a reusable catalyst for the coupling reaction of halophenols and arylboronic acids in aqueous media
Sakurai H, et al.
The Journal of Organic Chemistry, 67(8), 2721-2722 (2002)
Total synthesis of psammaplysenes A and B, naturally occurring inhibitors of FOXO1a nuclear export
Georgiades SN and Clardy J
Organic Letters, 7(19), 4091-4094 (2005)
Jiaoning Wang et al.
Electrophoresis, 26(12), 2402-2408 (2005-05-17)
In this paper we have presented a sensitive and rapid immunoassay (IA) method by capillary electrophoresis with an enhanced chemiluminescence detection system (CE-CL) based on the catalytic effects of horseradish peroxidase (HRP) on the luminol-hydrogen peroxide reaction. The conditions for
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service