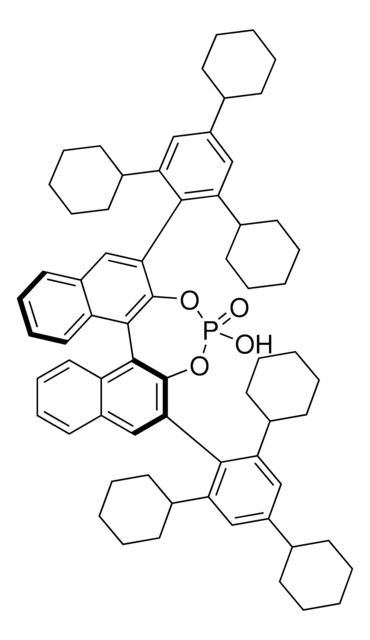
907502
Potassium 5-bromo-1H-indole-1-carbodithioate
≥95%
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C9H5BrKNS2
Molecular Weight:
310.28
UNSPSC Code:
12352101
NACRES:
NA.22
Recommended Products
Assay
≥95%
form
powder or crystals
reaction suitability
reaction type: Photocatalysis
reagent type: catalyst
mp
179-185 °C
storage temp.
2-8°C
Application
Potassium 5-bromo-1H-indole-1-carbodithioate is an organic photocatalyst shown to activate alkyl electrophiles via an SN2 pathway.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Photochemical generation of radicals from alkyl electrophiles using a nucleophilic organic catalyst.
Bertrand Schweitzer-Chaput et al.
Nature chemistry, 11(2), 129-135 (2018-12-05)
Chemists extensively use free radical reactivity for applications in organic synthesis, materials science, and life science. Traditionally, generating radicals requires strategies that exploit the bond dissociation energy or the redox properties of the precursors. Here, we disclose a photochemical catalytic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![10-Ethyl-3,7,8-trimethyl-benzo[g]pteridine-2,4(3H,10H)-dione ≥95%](/deepweb/assets/sigmaaldrich/product/structures/610/004/664b92ed-0ce1-4e66-9f3e-d1cc53f1bc9a/640/664b92ed-0ce1-4e66-9f3e-d1cc53f1bc9a.png)