724440
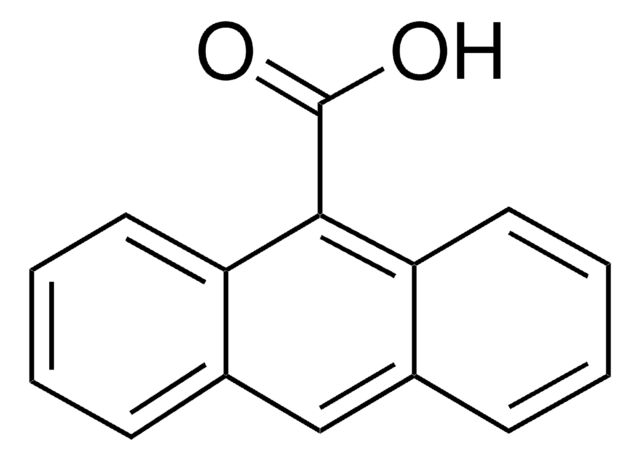
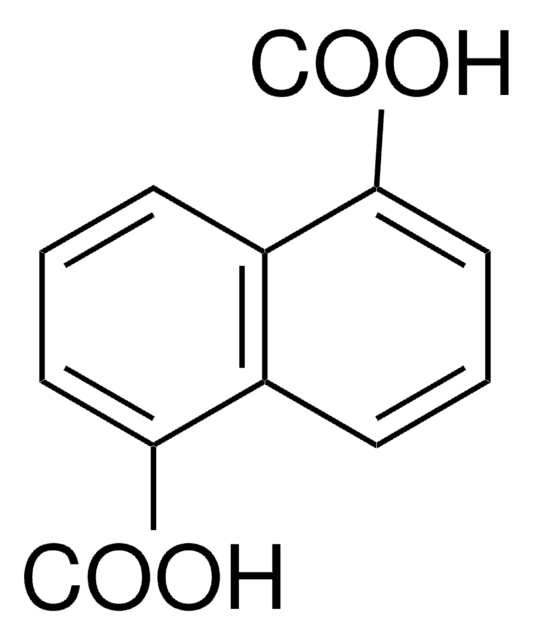
9,10-Anthracenedicarboxylic acid
95%
Synonym(s):
H2ADC
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C16H10O4
CAS Number:
Molecular Weight:
266.25
MDL number:
UNSPSC Code:
26111700
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
95%
form
solid
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
>300 °C
greener alternative category
, Enabling
SMILES string
OC(=O)c1c2ccccc2c(C(O)=O)c3ccccc13
InChI
1S/C16H10O4/c17-15(18)13-9-5-1-2-6-10(9)14(16(19)20)12-8-4-3-7-11(12)13/h1-8H,(H,17,18)(H,19,20)
InChI key
FDFGHPKPHFUHBP-UHFFFAOYSA-N
General description
9,10-Anthracenedicarboxylic acid (H2L) is an anthracene based dicarboxylic compound, which has a larger conjugating π-system that enables the development of fluorescent materials. It has interesting magnetic and luminescent properties. It can be used as a bridging carboxylic acid ligand with a steric bulk due to the presence of its anthracene ring.
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for energy efficiency. Find details here.
Application
H2L can be potentially used in the formation of highly porous nanotubular and ultramicroporous metal organic frameworks (MOFs) for greener applications like hydrogen storage, methane storage and gas separation.
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Cadmium (II) and lanthanum (III) coordination architectures with anthracene-9, 10-dicarboxylate: Crystal structures and photoluminescent properties
Wang J, et al.
Inorgorganica Chimica Acta, 385(6), 58-64 (2012)
Hisato Matsumoto et al.
Organic & biomolecular chemistry, 15(31), 6575-6583 (2017-07-28)
We report anthracene-diurea compounds which behave as anion sensors based on the fluorescence emission regulated by the substitution position on the anthracene ring. Anthracene-diurea compounds exhibit different excited-state intermolecular proton transfer (ESIPT) reactions depending on the pattern of the substituents.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service