721352
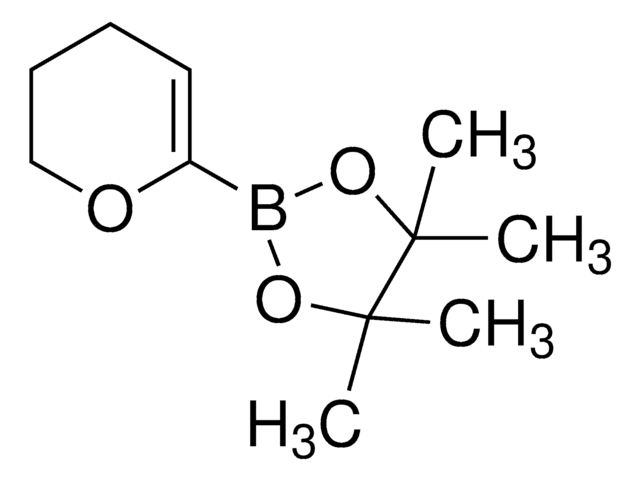
3,6-Dihydro-2H-pyran-4-boronic acid pinacol ester
97%
Synonym(s):
2-(3,6-Dihydro-2H-pyran-4-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C11H19BO3
CAS Number:
Molecular Weight:
210.08
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
63-67 °C
functional group
ether
storage temp.
2-8°C
SMILES string
CC1(C)OB(OC1(C)C)C2=CCOCC2
InChI
1S/C11H19BO3/c1-10(2)11(3,4)15-12(14-10)9-5-7-13-8-6-9/h5H,6-8H2,1-4H3
InChI key
DOSGEBYQRMBTGS-UHFFFAOYSA-N
Application
3,6-Dihydro-2H-pyran-4-boronic acid pinacol ester can be used:
- To prepare 1,2-dihydro-2-oxopyridine based endocannabinoid system (ECS) modulators.
- As an intermediate in the synthesis of embryonic ectoderm development (EED) inhibitors.
- To prepare pyrrolotriazine based IRAK4 inhibitors.
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
EEDi-5285: An Exceptionally Potent, Efficacious, and Orally Active Small-Molecule Inhibitor of Embryonic Ectoderm Development
Rej RK, et al.
Journal of medicinal chemistry, 63(13), 7252-7267 (2020)
Articles
The synthesis of biaryl compounds via the Suzuki–Miyaura coupling reaction has become more commonplace now that many arylboronic acids are readily available.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)