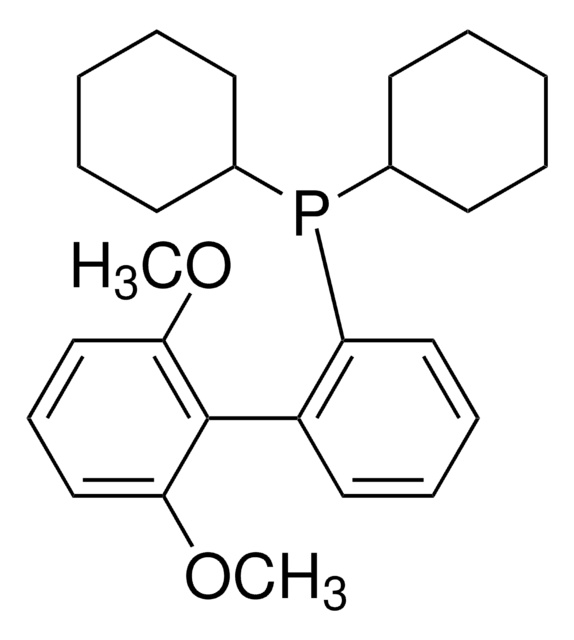
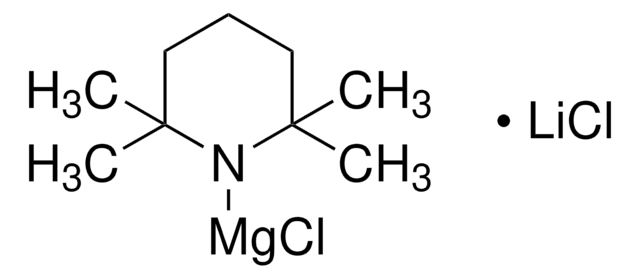
656984
Isopropylmagnesium chloride lithium chloride complex solution
1.3 M in THF
Synonym(s):
Turbo Grignard
About This Item
Recommended Products
reaction suitability
reaction type: Grignard Reaction
Quality Level
100
200
concentration
1.3 M in THF
density
0.951 g/mL at 25 °C
SMILES string
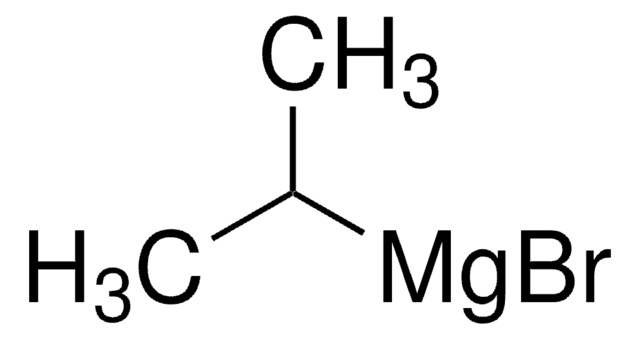
[Li]Cl.CC(C)[Mg]Cl
InChI
1S/C3H7.2ClH.Li.Mg/c1-3-2;;;;/h3H,1-2H3;2*1H;;/q;;;2*+1/p-2
InChI key
CWTUREABAILGIK-UHFFFAOYSA-L
Related Categories
Application
Used for:
- Preparation of functionalized acyclic alkenyl magnesium reagents/preparation of 1,2-diketones
- Preparation of cyclic alkenyl and dienyl magnesium reagents
- Preparation of functionalized triazenes/preparation of carbazoles
- Regioselective functionalization of trisubstituted pyridines
- Electrophilic amination for preparation of amines
- Preparation of Grignards reagents in the presence of masked ketones and aldehydes (using readily deprotectable silylated cyanohydrins)
- Preparation of Benzylic Grignards via Sulfur-Magnesium exchange
- Functionalization via I-Mg Exchange of unprotected aromatic and heteroaromatic carboxylic acids
- Functionalization via I-Mg Exchange of unprotected imidazoles
- Pyridyne formation
- Functionalization of cyclopentene derivatives
- Preparation of 2,3-functionalized furans, benzofurans, and thiophenes
- Stereoselective synthesis of substituted cyclopropanes
Packaging
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3
Target Organs
Central nervous system, Respiratory system
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
1.4 °F
Flash Point(C)
-17 °C
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Selective Metalations using i-PrMgCl·LiCl and s-BuMgCl·LiCl
Transformative reagents for selective metalation, deprotonation and nucleophilic additions have allowed for unprecedented selective converstions to reactive intermediates within a molecule which contains sensative functionalities under mild reactions.
We present an article concerning Reagents for Selective Metalation, Deprotonation, and 1,2-Additions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![[1,3-Bis(diphenylphosphino)propane]dichloronickel(II)](/deepweb/assets/sigmaaldrich/product/structures/844/065/af07f787-c6a3-4a6e-a22b-47a933c73978/640/af07f787-c6a3-4a6e-a22b-47a933c73978.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)