All Photos(1)
About This Item
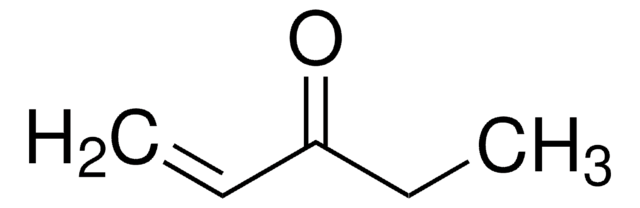
Linear Formula:
H2C=CHC6H10OH
CAS Number:
Molecular Weight:
126.20
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.478 (lit.)
bp
74 °C/19 mmHg (lit.)
density
0.942 g/mL at 25 °C (lit.)
functional group
hydroxyl
SMILES string
OC1(CCCCC1)C=C
InChI
1S/C8H14O/c1-2-8(9)6-4-3-5-7-8/h2,9H,1,3-7H2
InChI key
ZXKHOVDDJMJXQP-UHFFFAOYSA-N
General description
1-Vinyl cyclohexanol, also known as 1-vinyl-1-cyclohexanol, is a tertiary allylic alcohol. It can be synthesized from cyclohexanone and vinyl chloride. It can undergo transition-metal-free tandem allylic borylation in the presence of B2pin [bis(pinacolato)diboron], Cs2CO3, THF and MeOH to yield triborated product. Pd-fullerite catalysts have been prepared which effectively catalyzes the hydrogenation of 1-ethynyl-1-cyclohexanol to 1-vinyl-1-cyclohexanol.
Application
1-Vinyl cyclohexanol may be used to synthesize:
- 1-vinyl-1-cyclohexene
- 1-vinyl-1-cyclohexylacrylate
- cyclohexylideneacetaldehyde
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
158.0 °F - closed cup
Flash Point(C)
70 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of spirocyclic butenolides by ring closing metathesis.
Albrecht U and Langer P.
Tetrahedron, 63(22), 4648-4654 (2007)
One-pot rapid low-cost synthesis of Pd-fullerite catalysts.
Chong LC, et al.
Journal of Materials Chemistry, 18(40), 4808-4813 (2008)
Lewis Acid Promoted Oxidative Rearrangement of Tertiary Allylic Alcohols with the PhIO/TEMPO System.
Vatele JM.
Synlett, 12, 1785-1788 (2008)
Transition-Metal-Free Borylation of Allylic and Propargylic Alcohols.
Miralles N, et al.
Angewandte Chemie (International Edition in English), 128(13), 4375-4379 (2016)
Dimerization of conjugated cyclodienes.
Suga K, et al.
Canadian Journal of Chemistry, 45(9), 933-937 (1967)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service