468045
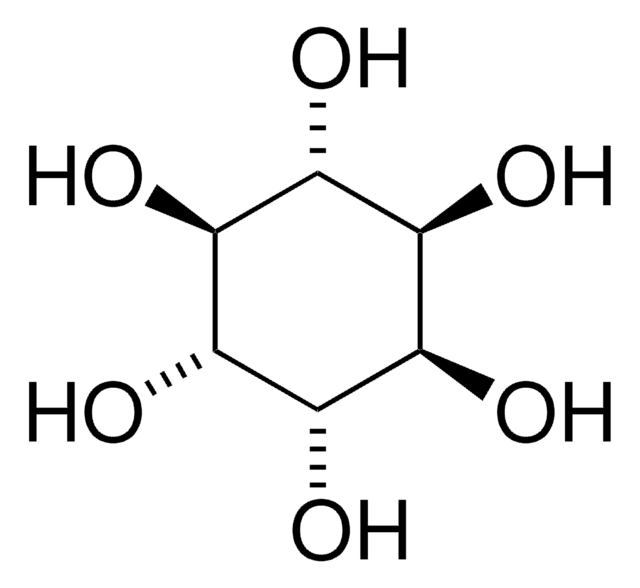
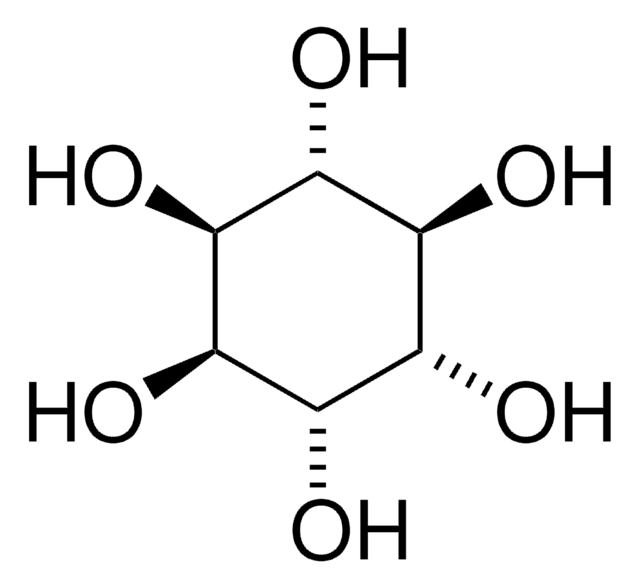
D-(+)-chiro-Inositol
95%
Synonym(s):
1,2,4/3,5,6-Hexahydroxycyclohexane
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C6H12O6
CAS Number:
Molecular Weight:
180.16
EC Number:
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
solid
optical activity
[α]23/D +60°, c = 1.2 in H2O
optical purity
ee: 99% (GLC)
mp
230 °C (lit.)
storage temp.
2-8°C
SMILES string
O[C@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@H](O)[C@@H]1O
InChI
1S/C6H12O6/c7-1-2(8)4(10)6(12)5(11)3(1)9/h1-12H/t1-,2-,3-,4-,5+,6+/m0/s1
InChI key
CDAISMWEOUEBRE-LKPKBOIGSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
The inositols and their phosphates have been used in the development of metabolically stable insulin mediators, inhibitors, and modulators of important metabolic functions such as glycolysis. Inositols are stable to degradative enzymes in vivo because they lack a hydrolytically labile glycosidic linkage.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Bellington, D.C.
Chemical Society Reviews, 18, 83-83 (1989)
Recent advances in the chemistry and biochemistry of inositol phosphates of biological interest.
B V Potter
Natural product reports, 7(1), 1-24 (1990-02-01)
Modern Methods of Monosaccharide Synthesis from Non-Carbohydrate Sources.
Tomas Hudlicky et al.
Chemical reviews, 96(3), 1195-1220 (1996-05-09)
Hudlicky, T. Cebulak, M.
Cyclitols and Their Derivatives. A Handbook of Physical, Spectral, and Synthetic Data (1993)
Hudlicky, T. et al.
Synthesis, 897-897 (1996)
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 468045-1G | 4061826696439 |
| 468045-100MG | 4061832358390 |
| 468045-25MG |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service