All Photos(1)
About This Item
Linear Formula:
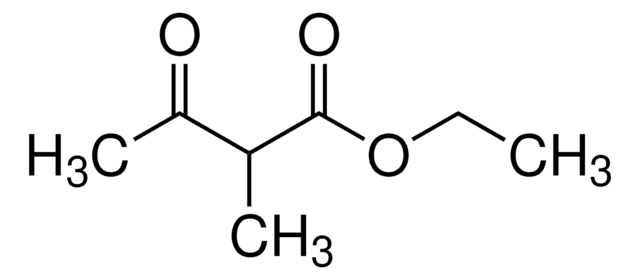
CH3COCH(C2H5)COOCH3
CAS Number:
Molecular Weight:
144.17
Beilstein:
1754068
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97.0% (GC)
refractive index
n20/D 1.423
density
1.010 g/mL at 20 °C (lit.)
functional group
ester
ketone
SMILES string
CCC(C(C)=O)C(=O)OC
InChI
1S/C7H12O3/c1-4-6(5(2)8)7(9)10-3/h6H,4H2,1-3H3
InChI key
YXLVLOWNJCOOAU-UHFFFAOYSA-N
General description
Methyl 2-ethylacetoacetate participates in the preparation of 5,6-disubstituted-1,3-dioxine-4-one.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Nils Pemberton et al.
Organic letters, 8(5), 935-938 (2006-02-24)
Polycyclic ring-fused 2-pyridones (5a-e and 9a-e) have been prepared via a microwave-assisted acyl-ketene imine cyclocondensation. Starting from 3,4-dihydroisoquinolines (4a-b) or 3,4-dihydroharman (8), fused 2-pyridones could be prepared in a one-step procedure. By using either Meldrum's acid derivatives (1a-d) or 1,3-dioxine-4-ones
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service