All Photos(1)
About This Item
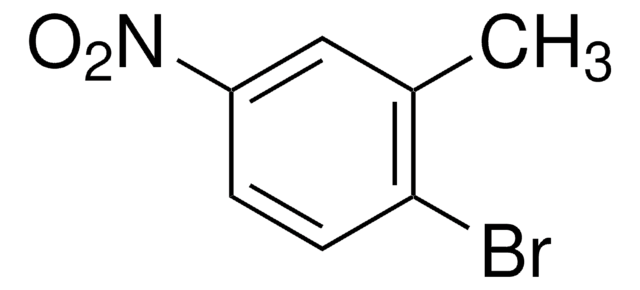
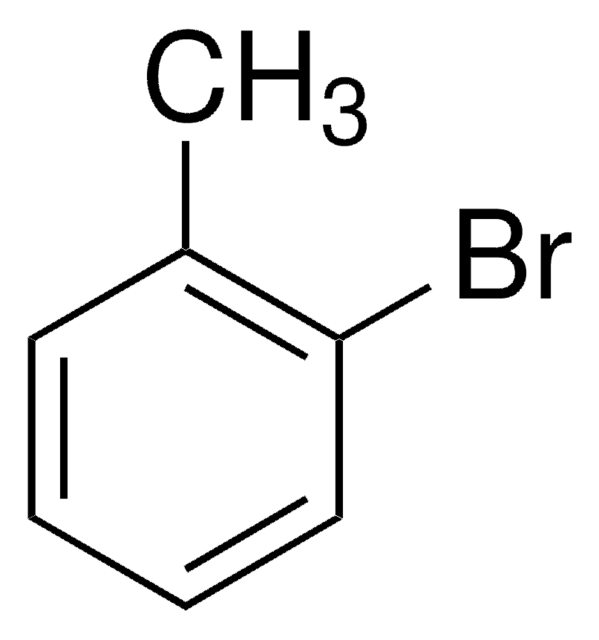
Linear Formula:
CH3C6H3(NO2)Br
CAS Number:
Molecular Weight:
216.03
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
bp
130 °C/12 mmHg (lit.)
mp
45-48 °C (lit.)
solubility
water: insoluble(lit.)
functional group
bromo
nitro
SMILES string
Cc1ccc(Br)cc1[N+]([O-])=O
InChI
1S/C7H6BrNO2/c1-5-2-3-6(8)4-7(5)9(10)11/h2-4H,1H3
InChI key
KZNXALJXBRSMFL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
4-Bromo-2-nitrotoluene is a nitrotoluene derivative. It can be synthesized by the regioselective bromination of o-nitrotoluene.
Application
4-Bromo-2-nitrotoluene may be used as a starting material in the synthesis of the following:
- 4-bromo-2-nitrobenzylidene
- 4-bromo-2-nitrobenzaldehyde
- 4-bromo-2-chlorotoluene
- 4-bromo-2-nitrobenzoic acid by oxidation
- 6-bromoindole by Batcho-Leimgruber indole synthesis
- 3-(4-bromo-2-nitrophenyl)-2-[2-(tert-butyldimethylsilanyloxy)ethyl]-N-(2,4-dichloro-6-iodophenyl)-N-methoxymethylacrylamide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Tyrian purple: 6, 6'-dibromoindigo and related compounds.
Cooksey CJ.
Molecules (Basel), 6(9), 736-769 (2001)
Olga V Barykina et al.
Organic letters, 12(11), 2664-2667 (2010-05-08)
The synthesis of (+/-)-eusynstyelamide A has been accomplished in six steps in 13% overall yield from 6-bromoindole, methyl glycidate, and Boc-protected agmatine. If oxygen is carefully excluded from the reaction, the key NaOH-catalyzed aldol dimerization of the alpha-ketoamide proceeded efficiently
Jae Hong Seo et al.
The Journal of organic chemistry, 71(23), 8891-8900 (2006-11-04)
An efficient synthetic strategy for installation of the two vicinal quaternary carbon centers of the communesins is reported. Key steps include the O-allylation/Claisen rearrangement of spirolactone systems, which are formed by tandem intramolecular Heck cyclization/carbonylation. Substituent and solvent effects on
The bromo-2-nitrobenzoic acids.
Erickson JLE, et al.
Journal of the American Chemical Society, 74(22), 5621-5623 (1952)
A Simple, Safe and Efficient Synthesis of Tyrian Purple (6, 6'-Dibromoindigo).
Wolk JL and Frimer AA.
Molecules (Basel), 15(8), 5561-5580 (2010)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
