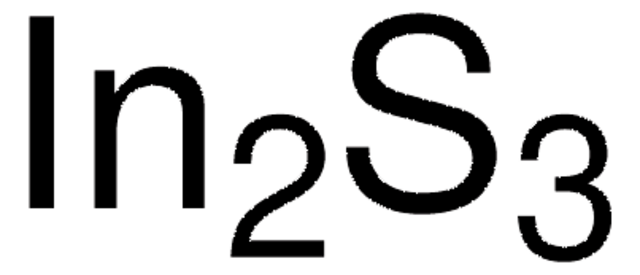410527
Gallium(III) sulfide
99.99% trace metals basis
Synonym(s):
Gallium sesquisulfide GaS
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
Ga2S3
CAS Number:
Molecular Weight:
235.64
EC Number:
MDL number:
UNSPSC Code:
12352300
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
99.99% trace metals basis
reaction suitability
reagent type: catalyst
core: gallium
density
3.65 g/mL at 25 °C (lit.)
SMILES string
S=[Ga]S[Ga]=S
InChI
1S/2Ga.3S
InChI key
RCKBMGHMPOIFND-UHFFFAOYSA-N
General description
Ga2S3 possesses high energy density, electrochemical study of the compound was studied. Bacterial oxidation of Ga2S3 was investigated.
Application
Gallium(III) sulfide (Ga2S3) may be used in lithium secondary batteries.
Packaging
1 g packaged in glass bottles; 5 g packaged in poly bottles
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Gallium (III) sulfide as an active material in lithium secondary batteries
Senoh H, et al.
Journal of Power Sources, 196(13), 5631-5636 (2011)
A E Torma
Canadian journal of microbiology, 24(7), 888-891 (1978-07-01)
The bacterial oxidation of naturally occurring gallium-bearing chalcopyrite concentrate and a pure synthetic gallium (III) sulfide has been investigated at pH 1.8 and 35 degree C, using an active culture of Thiobacillus ferrooxidans. This oxidation process may proceed by direct
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service