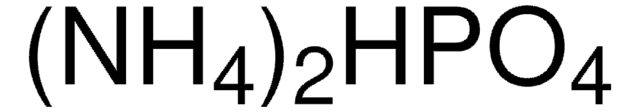379980
Ammonium phosphate dibasic
≥99.99% trace metals basis
Synonym(s):
Ammonium hydrogenphosphate, Diammonium hydrogenphosphate, di-Ammonium hydrogenphosphate (sec)
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(NH4)2HPO4
CAS Number:
Molecular Weight:
132.06
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
≥99.99% trace metals basis
form
crystalline
impurities
≤100.0 ppm Trace Metal Analysis
mp
155 °C (dec.) (lit.)
application(s)
battery manufacturing
SMILES string
N.N.OP(O)(O)=O
InChI
1S/2H3N.H3O4P/c;;1-5(2,3)4/h2*1H3;(H3,1,2,3,4)
InChI key
MNNHAPBLZZVQHP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Ammonium phosphate dibasic is widely used as a starting material to fabricate cathodes for Li-ion batteries. It can also be used to remove residual lithium from LiNiO2-based cathode materials.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Enhanced electrochemical properties of a LiNiO2-based cathode material by removing lithium residues with (NH4)2HPO4
Xunhui Xiong, et al.
Journal of Materials Chemistry, 2, 11691-11696 (2014)
Janet Sze-Jing Cheung et al.
International journal of hygiene and environmental health, 230, 113623-113623 (2020-09-16)
There are concerns in Yellowknife, Northwest Territories, Canada, about arsenic exposure due to past mining operations, particularly the former Giant Mine. The objective of this study was to characterize the risk of arsenic exposure and associated risk factors among the
Martin P Pothier et al.
Frontiers in microbiology, 9, 2310-2310 (2018-10-20)
Despite its high toxicity and widespread occurrence in many parts of the world, arsenic (As) concentrations in decentralized water supplies such as domestic wells remain often unquantified. One limitation to effective monitoring is the high cost and lack of portability
Nathan K Deed et al.
Applied microbiology and biotechnology, 89(5), 1537-1549 (2011-01-20)
Two deletion mutants expected to be defective in nitrogen catabolite repression (NCR) were constructed in a commercial wine yeast background M2: a ure2 mutant and a dal80 gzf3 double mutant. Wild-type and both mutant strains were fermented in Sauvignon Blanc
Catarina Barbosa et al.
International journal of food microbiology, 160(2), 87-93 (2012-11-28)
Sulphur-containing amino acids, cysteine and methionine, are generally found in very low concentrations in grape-juice. The objective of this study was to identify the effects of methionine on aroma compounds formation. Nitrogen source effects on growth, fermentative behaviour and aroma
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service