286281
Indole-3-acetamide
98%
Synonym(s):
3-Indolylacetamide, NSC 1969
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H10N2O
CAS Number:
Molecular Weight:
174.20
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
148-150 °C (lit.)
SMILES string
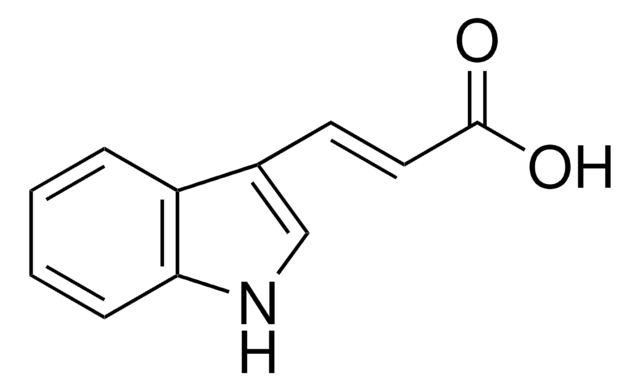
NC(=O)Cc1c[nH]c2ccccc12
InChI
1S/C10H10N2O/c11-10(13)5-7-6-12-9-4-2-1-3-8(7)9/h1-4,6,12H,5H2,(H2,11,13)
InChI key
ZOAMBXDOGPRZLP-UHFFFAOYSA-N
General description
Indole-3-acetamide is an auxin precursor.
Application
Indole-3-acetamide was used in the synthesis of [5.5.6.6]diazafenestrane skeleton and indole-3-acetic acid.
Reactant for the synthesis of:
- PET agent for imaging of protein kinase C
- A potential agent against Prion Disease
- Protein kinase C (PKC) inhibitor bisindolylmaleimide IV
- Glycogen synthase kinase-3ß (GSK-3ß) inhibitors
- Inhibitors of CaMKIId
- A VEGF inhibitor
- JAK3 inhibitors
- Inhibitors of NAD+-Dependent Histone Deacetylases
- Inhibitors of human adipocyte fatty acid-binding protein
- Cyclin-dependent kinase inhibitors
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Stephan Pollmann et al.
Phytochemistry, 70(4), 523-531 (2009-03-10)
Plants are suggested to produce their major growth promoting phytohormone, indole-3-acetic acid (IAA), via multiple redundantly operating pathways. Although great effort has been made and plenty of possible routes have been proposed based on experimental evidence, a complete pathway for
Casandra K Gutierrez et al.
Applied and environmental microbiology, 75(8), 2253-2258 (2009-02-17)
Strains of Vibrio spp. isolated from roots of the estuarine grasses Spartina alterniflora and Juncus roemerianus produce the phytohormone indole-3-acetic acid (IAA). The colorimetric Salkowski assay was used for initial screening of IAA production. Gas chromatography-mass spectroscopy (GC-MS) was then
Chuntao Yin et al.
Molecular plant-microbe interactions : MPMI, 27(3), 227-235 (2013-12-20)
The plant hormone indole-3-acetic acid (IAA) is best known as a regulator of plant growth and development but its production can also affect plant-microbe interactions. Microorganisms, including numerous plant-associated bacteria and several fungi, are also capable of producing IAA. The
Stephan Pollmann et al.
Phytochemistry, 62(3), 293-300 (2003-03-07)
Acylamidohydrolases from higher plants have not been characterized or cloned so far. AtAMI1 is the first member of this enzyme family from a higher plant and was identified in the genome of Arabidopsis thaliana based on sequence homology with the
Christian O Dimkpa et al.
Applied and environmental microbiology, 78(5), 1404-1410 (2012-01-03)
The beneficial bacterium Pseudomonas chlororaphis O6 produces indole-3-acetic acid (IAA), a plant growth regulator. However, the pathway involved in IAA production in this bacterium has not been reported. In this paper we describe the involvement of the indole-3-acetamide (IAM) pathway
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service