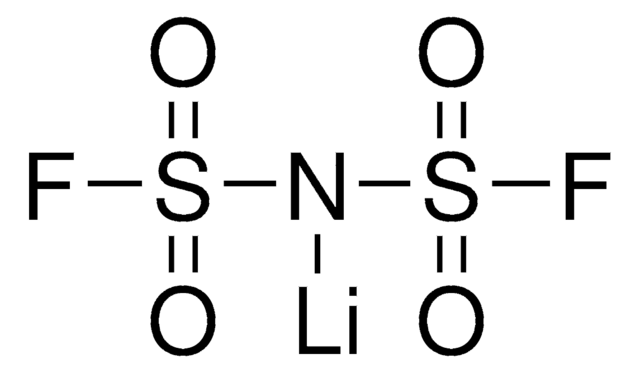282669
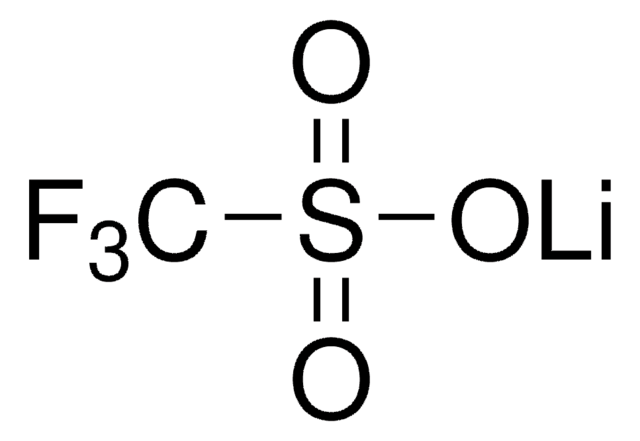
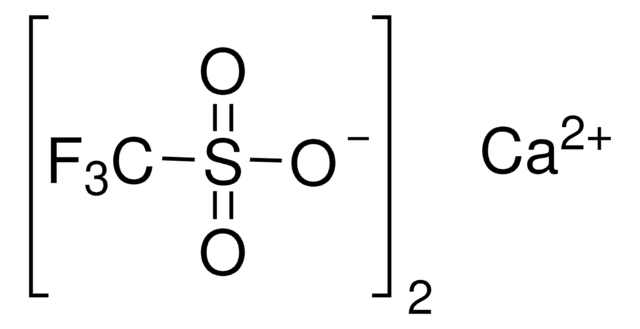
Lithium trifluoromethanesulfonate
96%
Synonym(s):
LiTf, Lithium triflate, Trifluoromethanesulfonic acid lithium salt
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CF3SO3Li
CAS Number:
Molecular Weight:
156.01
Beilstein:
4301818
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.22
grade:
for analytical purposes
form:
powder
Recommended Products
grade
for analytical purposes
Quality Level
Assay
96%
form
powder
mp
>300 °C (lit.)
functional group
fluoro
triflate
SMILES string
[Li+].[O-]S(=O)(=O)C(F)(F)F
InChI
1S/CHF3O3S.Li/c2-1(3,4)8(5,6)7;/h(H,5,6,7);/q;+1/p-1
InChI key
MCVFFRWZNYZUIJ-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Lithium trifluoromethanesulfonate can be used:
- As an electrolyte salt to determine the thermal stability of graphite anodes in Li-ion batteries.
- For the synthesis of solid polymer electrolytes with polyethylene oxide and chitosan, having potential applications in solid state batteries.
- As a source of triflate anion (CF3SO3−) to synthesize ionic liquids for further syntheses of conducting polymer electrolytes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
FTIR studies of chitosan acetate based polymer electrolytes.
Osman Z and Arof AK
Electrochimica Acta, 48(8), 993-999 (2003)
Ion conducting behaviour of polymer electrolytes containing ionic liquids.
Singh B and Sekhon SS
Chemical Physics Letters, 414(1-3), 34-39 (2005)
Solid polymer electrolytes based on polyethylene oxide and lithium trifluoro-methane sulfonate (PEO-LiCF3SO3): Ionic conductivity and dielectric relaxation.
Karan NK, et al.
Solid State Ionics, 179(19-20), 689-696 (2008)
The influence of lithium salt on the interfacial reactions controlling the thermal stability of graphite anodes.
Andersson AM, et al.
Electrochimica Acta, 47(12), 1885-1898 (2002)
Guangmin Zhou et al.
Nature communications, 6, 7760-7760 (2015-07-18)
Lithium-sulphur batteries with a high theoretical energy density are regarded as promising energy storage devices for electric vehicles and large-scale electricity storage. However, the low active material utilization, low sulphur loading and poor cycling stability restrict their practical applications. Herein
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 282669-100G | 4061826235720 |
| 282669-25G | 4061835554898 |
| 282669-5G |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service