262064
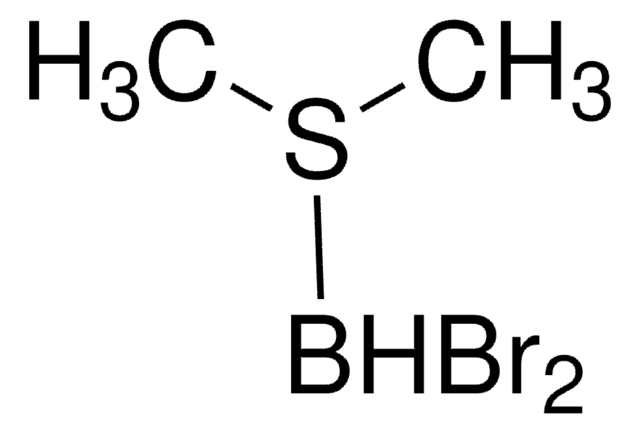
mono-Bromoborane methyl sulfide complex solution
1.0 M in methylene chloride
Synonym(s):
Bromo(dimethyl sulfide)borane, Bromo(dimethyl sulfide)dihydroboron
About This Item
Recommended Products
form
liquid
reaction suitability
reagent type: reductant
concentration
1.0 M in methylene chloride
density
1.347 g/mL at 25 °C
functional group
thioether
SMILES string
BBr.CSC
InChI
1S/C2H6S.BBrH2/c1-3-2;1-2/h1-2H3;1H2
InChI key
AQRNIOMHNXJPFD-UHFFFAOYSA-N
Related Categories
Application
- Substitution reactions for synthesis of N-heterocyclic carbene boranes with boron-heteroatom bonds
- Hydroboration reactions
- Regio- and chemoselective brominative cleavage of terminal epoxides
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3 - Water-react 1
Target Organs
Central nervous system
Supplementary Hazards
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 3
Flash Point(F)
50.0 °F
Flash Point(C)
10 °C
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
EU REACH Annex XVII (Restriction List)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service