All Photos(1)
About This Item
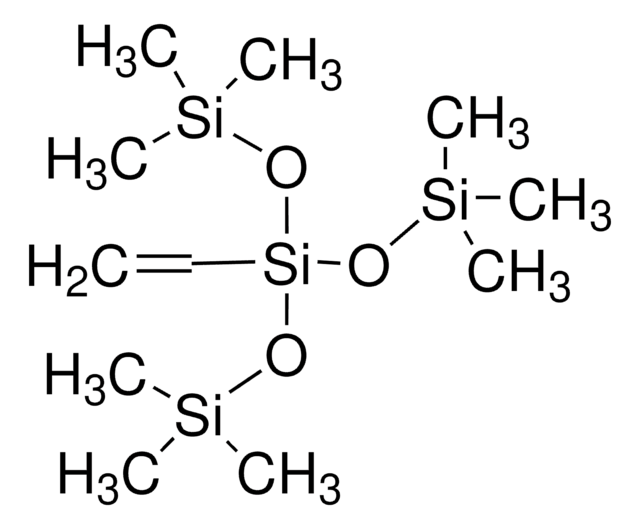
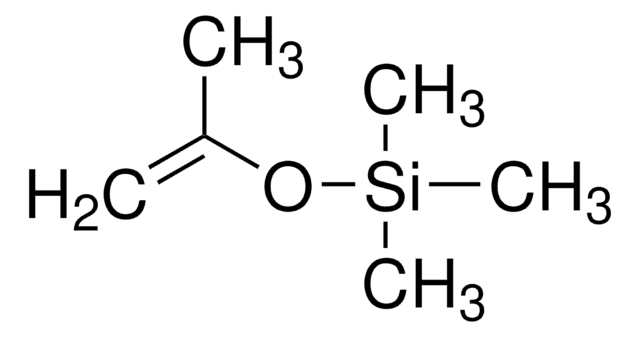
Linear Formula:
[(CH3)3SiO]2C=CHOSi(CH3)3
CAS Number:
Molecular Weight:
292.59
Beilstein:
1962905
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
liquid
refractive index
n20/D 1.420 (lit.)
bp
54-56 °C/0.1 mmHg (lit.)
density
0.886 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C[Si](C)(C)O\C=C(\O[Si](C)(C)C)O[Si](C)(C)C
InChI
1S/C11H28O3Si3/c1-15(2,3)12-10-11(13-16(4,5)6)14-17(7,8)9/h10H,1-9H3
InChI key
FCZGHPGTZRTDNN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Tris(trimethylsiloxy)ethylene has been used in:
- stereospecific synthesis of insecticide ajuqarin-IV
- microwave-assisted synthesis of 2-hydroxy-1-phenylethanone
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
136.4 °F - closed cup
Flash Point(C)
58 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Multienzymatic preparation of 3-[(1R)-1-hydroxyethyl] benzoic acid and (2< i> S</i>)-hydroxy (phenyl) ethanoic acid.
Gennaro PD, et al.
Tetrahedron Asymmetry, 21(15), 1885-1889 (2010)
Stereospecific total synthesis of ajugarin-IV.
Kende AS, et al.
Tetrahedron Letters, 23(17), 1751-1754 (1982)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![3-[Tris(trimethylsiloxy)silyl]propyl methacrylate contains MEHQ + HQ as stabilizer, 98%](/deepweb/assets/sigmaaldrich/product/structures/148/664/33ff5116-f264-4a64-824a-009c2ca5b2b3/640/33ff5116-f264-4a64-824a-009c2ca5b2b3.png)
![Tris[3-(trimethoxysilyl)propyl] isocyanurate technical grade](/deepweb/assets/sigmaaldrich/product/structures/239/690/c24b2d6d-4580-41dd-a3ec-77f7fcb9caaf/640/c24b2d6d-4580-41dd-a3ec-77f7fcb9caaf.png)