192805
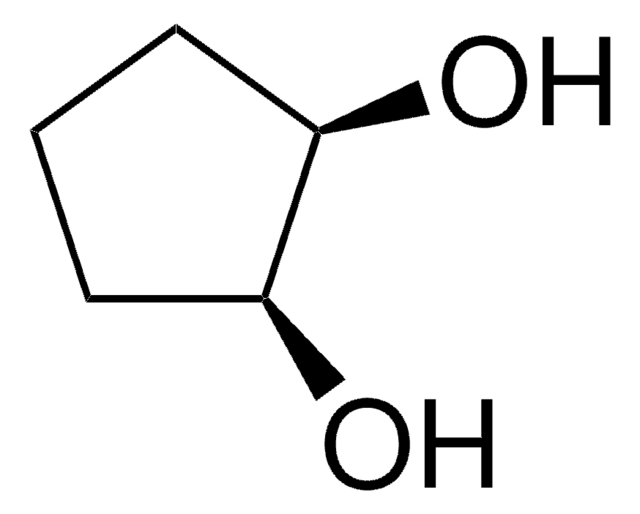
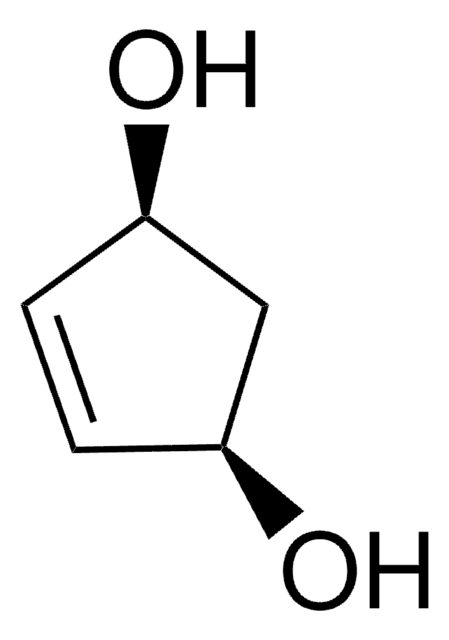
1,3-Cyclopentanediol, mixture of cis and trans
95%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C5H8(OH)2
CAS Number:
Molecular Weight:
102.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.484 (lit.)
bp
80-85 °C/0.1 mmHg (lit.)
mp
40 °C (lit.)
density
1.094 g/mL at 25 °C (lit.)
SMILES string
OC1CCC(O)C1
InChI
1S/C5H10O2/c6-4-1-2-5(7)3-4/h4-7H,1-3H2
InChI key
NUUPJBRGQCEZSI-UHFFFAOYSA-N
Related Categories
Application
1,3-Cyclopentanediol was used in the synthesis of 1,3-cyclopentanediol bis(4-methylbenzenesulfonate) and β-hydroxy-substituted cyclic carbonyl compounds. It was also used in direct enantiomer resolution of diols without derivatization on a chiral polysiloxane by capillary gas chromatography.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Direct asymmetric a-hydroxylation of 2-hydroxymethyl ketones.
Paju A, et al.
Tetrahedron, 58(36), 7321-7326 (2002)
Stepwise Introduction of p-Electron Cross-Conjugation: A Possible Access to [5] Radialenes?
Geneste F, et al.
The Journal of Organic Chemistry, 62(16), 5339-5343 (1997)
Direct resolution of enantiomeric diols by capillary gas chromatography on a chiral polysiloxane derived from (R, R)-tartramide.
Nakamura K, et al.
Analytical Chemistry, 62(5), 539-541 (1990)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service