178683
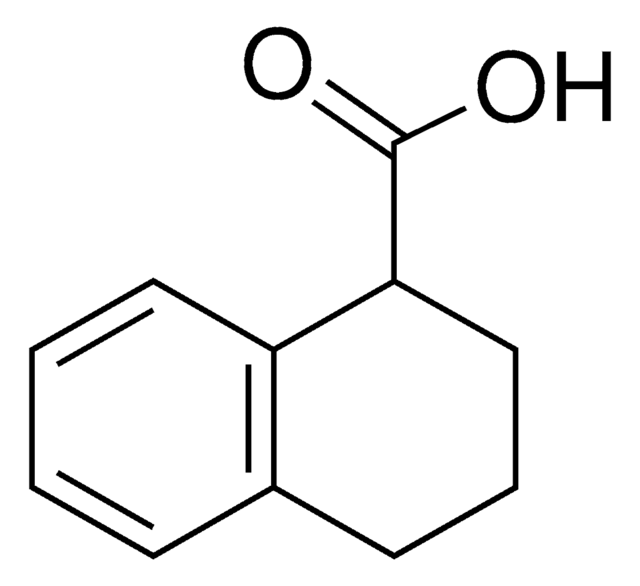
1,2,3,4-Tetrahydro-2-naphthoic acid
98%
Synonym(s):
Tetralin-2-carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H12O2
CAS Number:
Molecular Weight:
176.21
Beilstein:
2050435
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
mp
93-96 °C (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)C1CCc2ccccc2C1
InChI
1S/C11H12O2/c12-11(13)10-6-5-8-3-1-2-4-9(8)7-10/h1-4,10H,5-7H2,(H,12,13)
InChI key
NTAGXJQHJQUOOA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
R U Meckenstock et al.
Applied and environmental microbiology, 66(7), 2743-2747 (2000-07-06)
Anaerobic naphthalene degradation by a sulfate-reducing enrichment culture was studied by substrate utilization tests and identification of metabolites by gas chromatography-mass spectrometry. In substrate utilization tests, the culture was able to oxidize naphthalene, 2-methylnaphthalene, 1- and 2-naphthoic acids, phenylacetic acid
Beili Wang et al.
Environmental science & technology, 53(2), 1022-1030 (2018-12-18)
The nontargeted scanning chemical profiling approach has shown great potential to identify unknown pollutants or novel biological markers; however, the structure identification of unknown compounds is a challenge. In this study, a carboxyl-specific derivatization reagent, N-(4-aminomethylphenyl) pyridinium (AMPP), was coupled
Hazel M Girvan et al.
Journal of inorganic biochemistry, 188, 18-28 (2018-08-18)
The CYP152 family of cytochrome P450 enzymes (P450s or CYPs) are bacterial peroxygenases that use hydrogen peroxide to drive hydroxylation and decarboxylation of fatty acid substrates. We have expressed and purified a novel CYP152 family member - CYP152K6 from the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service