124168
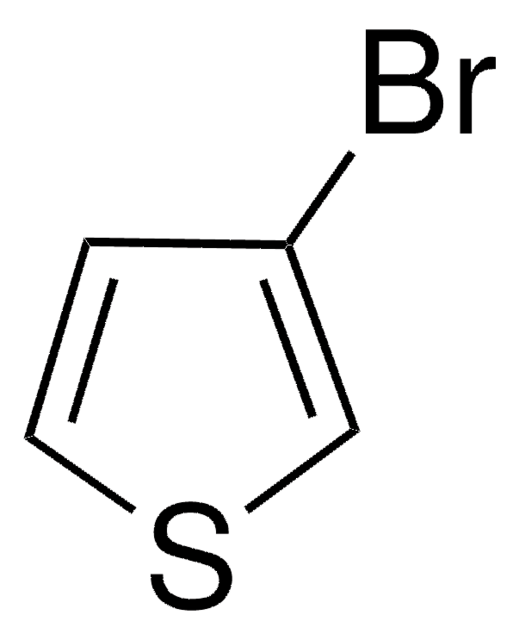
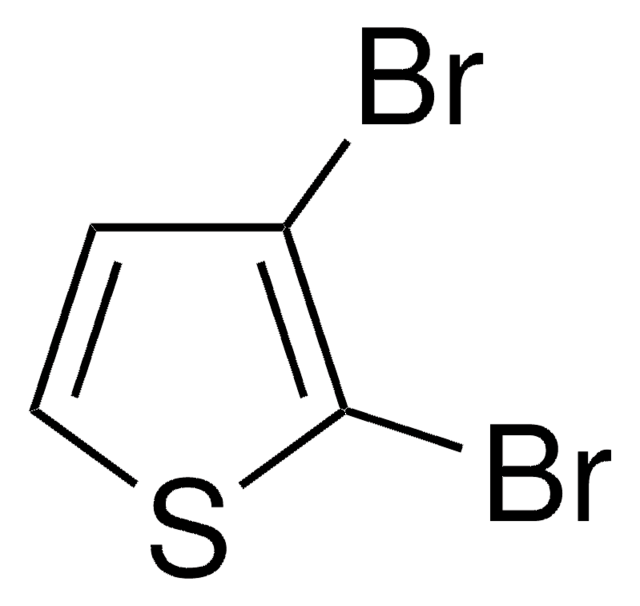
2-Bromothiophene
98%
Synonym(s):
2-Thienyl bromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H3BrS
CAS Number:
Molecular Weight:
163.04
Beilstein:
104663
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.586 (lit.)
bp
149-151 °C (lit.)
density
1.684 g/mL at 25 °C (lit.)
functional group
bromo
storage temp.
2-8°C
SMILES string
Brc1cccs1
InChI
1S/C4H3BrS/c5-4-2-1-3-6-4/h1-3H
InChI key
TUCRZHGAIRVWTI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Bromothiophene undergoes metalation-alkylation reaction with various electrophiles to form various 5-alkylated 2-bromo products. It undergoes coupling with 4-bromo allyl phenyl ether to form allyl phenyl thiophene ether, which is a novel potential dielectric material for organic thin film transistors.
Application
2-Bromothiophene has been used in electrochemical reduction of a number of mono- and dihalothiophenes at carbon cathodes in dimethylformamide containing tetramethylammonium perchlorate by cyclic voltammetry and controlled-potential electrolysis.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Oral - Eye Dam. 1 - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
125.6 °F - closed cup
Flash Point(C)
52 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synlett, 974-974 (2007)
R A Ingle et al.
The Journal of chemical physics, 147(1), 013914-013914 (2017-07-10)
The ultraviolet photochemistry of 2-bromothiophene (C
First example of base-promoted tandem alkylation-bromination of 2-bromothiophene via halogen dance process: a remarkable temperature effect.
Peyron C, et al.
Tetrahedron Letters, 46(19), 3315-3318 (2005)
Novel self assembled monolayers of allyl phenyl thiophene ether as potential dielectric material for organic thin film transistors.
Sathyapalan A, et al
Thin Solid Films, 516(16), 5645-5648 (2008)
Mohammad S. Mubarak et al.
The Journal of organic chemistry, 61(23), 8074-8078 (1996-11-15)
Cyclic voltammetry and controlled-potential electrolysis have been employed to probe the electrochemical reduction of a number of mono- and dihalothiophenes at carbon cathodes in dimethylformamide containing tetramethylammonium perchlorate. Reduction of 2-bromo-, 3-bromo-, 2-chloro-, 3-chloro-, and 2-iodothiophene gives rise to a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service