123188
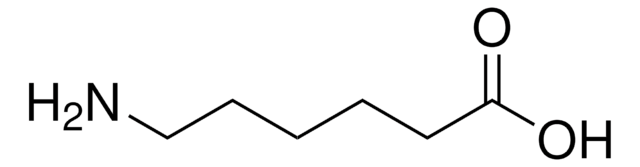
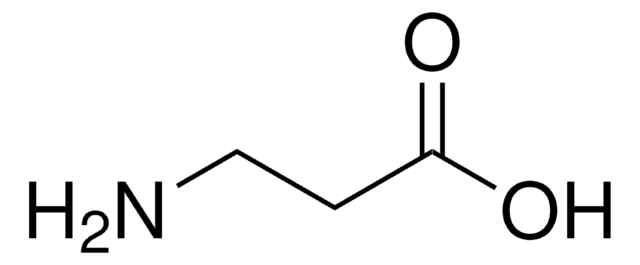
5-Aminovaleric acid
97%, for peptide synthesis
Synonym(s):
5-AVA, 5-Aminopentanoic acid, Homopiperidinic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
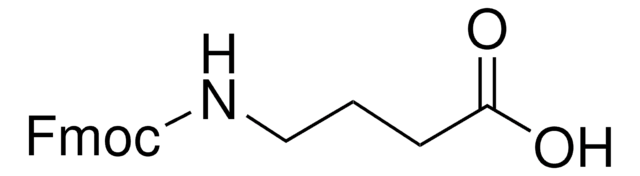
Linear Formula:
NH2(CH2)4CO2H
CAS Number:
Molecular Weight:
117.15
Beilstein:
906833
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
product name
5-Aminovaleric acid, 97%
Assay
97%
form
solid
reaction suitability
reaction type: solution phase peptide synthesis
mp
158-161 °C (lit.)
application(s)
peptide synthesis
SMILES string
NCCCCC(O)=O
InChI
1S/C5H11NO2/c6-4-2-1-3-5(7)8/h1-4,6H2,(H,7,8)
InChI key
JJMDCOVWQOJGCB-UHFFFAOYSA-N
Gene Information
human ... SLC15A1(6564)
Looking for similar products? Visit Product Comparison Guide
Application
5-Aminovaleric acid (5-AVA) is used:
- In the preparation of (5-AVA)x(MA)1-xPbI3, a perovskite for fabricating printable mesoscopic perovskite solar cell.
- As a spacer in the synthesis of rhenium and technetium-99m labeled insulin.
- To synthesize dipeptides that self-assemble to form nanotubes in the solid state as well as in solution over a wide range of pH.
- As a starting material in the total synthesis of an alkaloid, lycoposerramine Z.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A hole-conductor?free, fully printable mesoscopic perovskite solar cell with high stability.
Mei A, et al.
Science, 345(6194), 295-298 (2014)
Dipeptide Nanotubes, with N-Terminally Located ω-Amino Acid Residues, That are Stable Proteolytically, Thermally, and Over a Wide Range of pH.
Guha S, et al.
Chemistry of Materials, 20(6), 2282-2290 (2008)
cis-Decahydroquinolines via asymmetric organocatalysis: Application to the total synthesis of lycoposerramine Z.
Bradshaw B, et al.
Organic Letters, 15(2), 326-329 (2012)
Synthesis and characterization of rhenium and technetium-99m labeled insulin.
Sundararajan C, et al.
Journal of Medicinal Chemistry, 53(6), 2612-2621 (2010)
Sylvain Poujol et al.
Clinical chemistry, 49(11), 1900-1908 (2003-10-28)
We developed gradient HPLC methods for quantification of the antimitotic drug irinotecan (CPT-11) and its four metabolites, SN-38, SN-38 G, 7-ethyl-10-[4-N-(5-aminopentanoic acid)-1-piperidino]-carbonyloxycamptothecin (APC), and 7-ethyl-10-[4amino-1-piperidino]-carbonyloxycamptothecin (NPC), as the sum of the lactone and carboxylate forms, in human plasma and saliva.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service