W0769
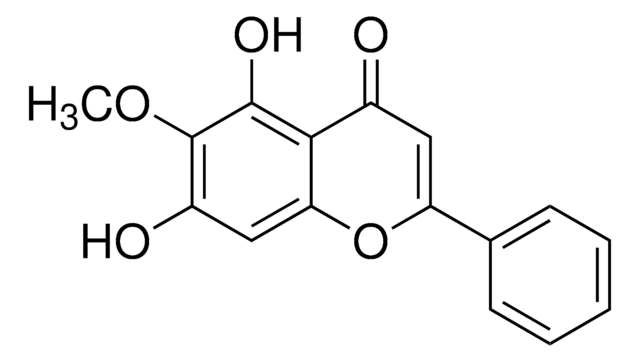
Wogonin hydrate
≥98% (HPLC), solid
Synonym(s):
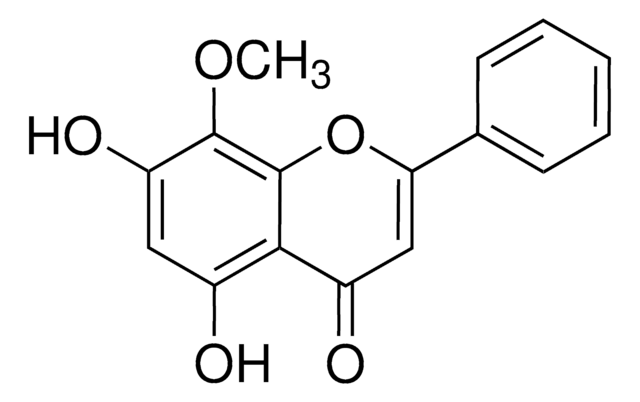
5,7-Dihydroxy-8-methoxyflavone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C16H12O5 · xH2O
CAS Number:
Molecular Weight:
284.26 (anhydrous basis)
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Assay
≥98% (HPLC)
form
solid
solubility
DMSO: ≥20 mg/mL
storage temp.
2-8°C
SMILES string
O.COc1c(O)cc(O)c2C(=O)C=C(Oc12)c3ccccc3
InChI
1S/C16H12O5.H2O/c1-20-15-12(19)7-10(17)14-11(18)8-13(21-16(14)15)9-5-3-2-4-6-9;/h2-8,17,19H,1H3;1H2
InChI key
XLCSETHCROERAU-UHFFFAOYSA-N
General description
Wogonin is a flavonoid component of Scutellaria baicalensis Georgi roots.
Application
Wogonin hydrate has been used to enhance the cytotoxicity of oxaliplatin in gastric cancer cells and zebrafish xenograft model and to test its effect on the expression and secretion of adiponectin in mature 3T3-L1 adipocytes.
Biochem/physiol Actions
Wogonin co-treatment enhances apoptosis and antitumor potential of chemotherapeutic drug, oxaliplatin in gastric cancer cells. It also improves the efficacy of fluorouracil and paclitaxel. Wogonin anti-oxidative functionality may be useful in treating neurodegenerative and inflammatory diseases.
Wogonin is an anti-inflammatory agent and COX-2 inhibitor, which inhibits the induction of both iNOS and COX-2.
Wogonin is an anti-inflammatory agent and COX-2 inhibitor, which inhibits the induction of both iNOS and COX-2. Wogonin inhibits COX-2 (IC50 = 46 μM) without affecting COX-1. Wogonin inhibits iNOS induction and thus inhibts activation-induced C6 glial cell death. Specifically, Wogonin inhibits NF-kappaB-mediated iNOS induction.
Features and Benefits
This compound is featured on the Nitric Oxide Synthases page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Fei Wang et al.
Toxicology, 305, 10-19 (2012-12-19)
Wogonin, a naturally occurring monoflavonoid extracted from the root of Scutellaria baicalensis Georgi, has been reported for its anti-oxidant activity. However, it is still unclear whether wogonin can inhibit oxidant-induced vascular permeability. In this study, we evaluated the effects of
Yan Zhong et al.
Molecular carcinogenesis, 52(10), 824-834 (2012-05-18)
Acquired resistance to doxorubicin (DOX) is a serious therapeutic problem in breast cancer patients. In this study, we investigated whether nuclear factor erythroid 2-related factor 2 (Nrf2) was associated with drug resistant in DOX resistant MCF-7 (MCF-7/DOX) cells, and if
Meng Zhang et al.
Life sciences, 92(1), 55-62 (2012-11-13)
Wogonin is one of the major constituents derived from Scutellaria Baicalensis, which has been reported to inhibit cell growth and/or induce apoptosis in various cancer cell lines. We aim to investigate the anticancer effects and associated mechanisms of wogonin on
Xiuming Song et al.
Toxicology and applied pharmacology, 271(2), 144-155 (2013-05-28)
Wogonin, a plant-derived flavone, has been shown recently to have antitumor effects. However, the mechanisms that wogonin inhibits tumor angiogenesis are not well known. In this study, we investigated the effects of wogonin on expression of hypoxia-inducible factor-1α (HIF-1α) and
Yoshiyuki Kimura et al.
Phytomedicine : international journal of phytotherapy and phytopharmacology, 20(3-4), 328-336 (2012-12-12)
Tumor growth and metastasis are associated with angiogenesis and lymphangiogenesis through the production of vascular endothelial growth factor (VEGF) or VEGF-C in tumors, and the phosphorylation of VEGF receptor (VEGFR)-2 or VEGFR-3 in vascular endothelial cells or lymphatic endothelial cells
Articles
Sigma-Aldrich offers many products related to nitric oxide synthases for your research needs.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service