S9415
Streptavidin−Maleimide from Streptomyces avidinii
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
extent of labeling
4-10 mol maleimide per mol Streptavidin
Quality Level
200
400
suitability
suitable for direct conjugation to compounds containing free sulfhydryl groups
storage temp.
−20°C
General description
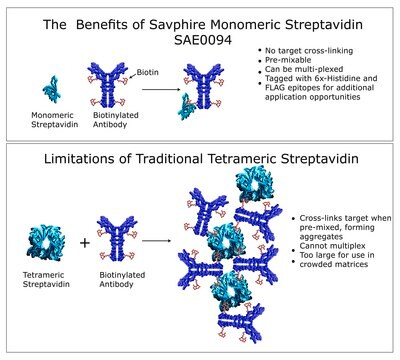
Streptavidin conjugated compounds may be used in avidin/strepavidin:biotin-based detection systems.
Streptavidin is a protein produced by the bacterium Streptomyces avidinii. It has four binding sites for biotin, as does avidin. Both have been used extensively as probes in immunochemical systems, conjugated to antibodies, enzymes or fluorochromes. However, avidin is highly basic (pI ~ 10.5), compared to streptavidin (pI ~ 5-6). Avidin has a slightly higher binding capacity for biotin than streptavidin does, but it has glycosylated side chains which give higher backgrounds in blotting techniques than does streptavidin, which is not a glycoprotein. Streptavidin is more resistant than avidin to dissociation into subunits by guanidinium chloride.
Application
Maleimide Activated Streptavidin is used to prepare streptavidin-protein conjugates with proteins, peptides and other molecules that contain a free sulfhydryl (-SH) group. Activated streptavidin presents an available maleimide group that reacts with sulfhydryl-containing molecules.
Physical form
Lyophilized powder containing sodium citrate.
Preparation Note
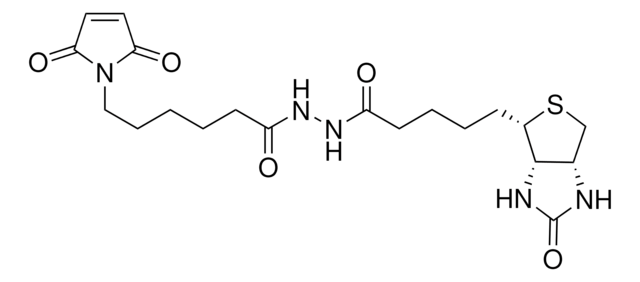
Streptavidin, from Streptomyces avidinii, activated with maleimidocaproic acid N-hydroxysuccinimide ester.
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
B Mygind et al.
European journal of biochemistry, 59(2), 365-372 (1975-11-15)
1. The transport of pyrimidine mucleosides into cells of Escherichis coli has been investigated in mutant strains which cannot metabolize these nucleosides. Such cells transport and concentrate purimidine mucleosides several hindredfold. 2. The transport is inhibited by energy poisons and
Abraham Wolcott et al.
The journal of physical chemistry. B, 110(11), 5779-5789 (2006-03-17)
Quantum dots (QDs) have been increasingly used in biolabeling recently as their advantages over molecular fluorophores have become clear. For bioapplications QDs must be water-soluble and buffer stable, making their synthesis challenging and time-consuming. A simple aqueous synthesis of silica-capped
New procedures for preparation and isolation of conjugates of proteins and a synthetic copolymer of D-amino acids and immunochemical characterization of such conjugates.
F T Liu et al.
Biochemistry, 18(4), 690-693 (1979-02-20)
Kitagawa, T., et al.
Chemical & Pharmaceutical Bulletin, 29(4), 1131-1131 (1981)
R J Duncan et al.
Analytical biochemistry, 132(1), 68-73 (1983-07-01)
A synthesis of the N-hydroxysuccinimide ester of S-acetylthioacetic acid is described. This material is stable when stored dry and has advantages over the currently available reagents used to introduce sulfhydryl groups into a variety of proteins. Proteins modified with this
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service