R1131
Anti-Rabbit IgG (whole molecule) antibody produced in goat
whole antiserum
Synonym(s):
Goat Anti-Rabbit IgG (whole molecule)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
biological source
goat
conjugate
unconjugated
antibody form
whole antiserum
antibody product type
secondary antibodies
clone
polyclonal
contains
15 mM sodium azide
technique(s)
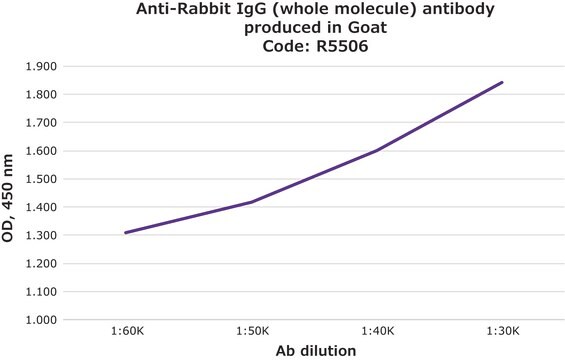
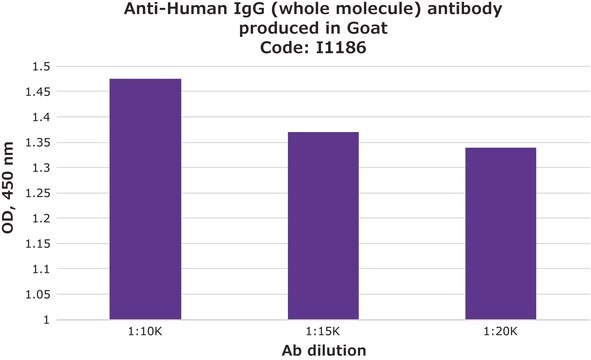
indirect ELISA: 1:150,000
quantitative precipitin assay: 15.0 mg/mL
shipped in
dry ice
storage temp.
−20°C
target post-translational modification
unmodified
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
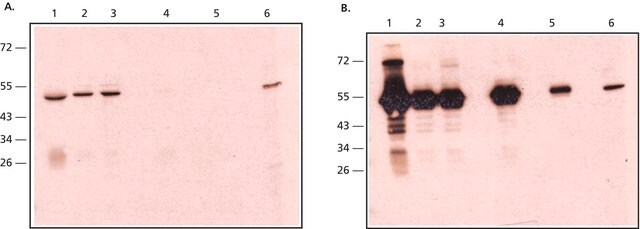
Anti-Rabbit IgG is developed in goat using rabbit IgG isolated from normal rabbit serum as the immunogen. IgG is present in large quantities in the human serum. It constitutes about 10-20% of the plasma proteins. IgG is composed of glycoproteins, out of which it is 82-96% proteins and 4-18% carbohydrates. It consists of four sub-classes i.e IgG1, IgG2, IgG3, and IgG4. IgG is composed of four polypeptide chains-two heavy chains (γchains) and two light chains (κ or λ chains) which are linked by inter-chain disulfide bonds. The heavy chains consist of a N-terminal variable domain (VH) and three constant domains (CH1, CH2, CH3). A hinge region exists between the CH1 and CH2 region. The light chains have one N-terminal variable domain (VL) and one constant domain (CL). The heavy and the light chains are linked at VH and CH1 domain to form the Fab arm (Fragment antigen binding). The antigen binds to the V regions of the antibody.
Application
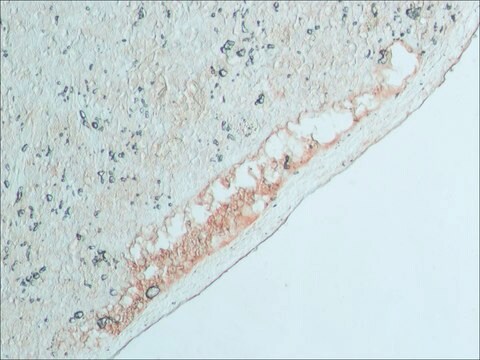
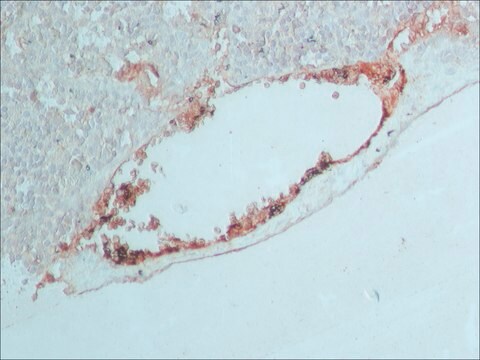
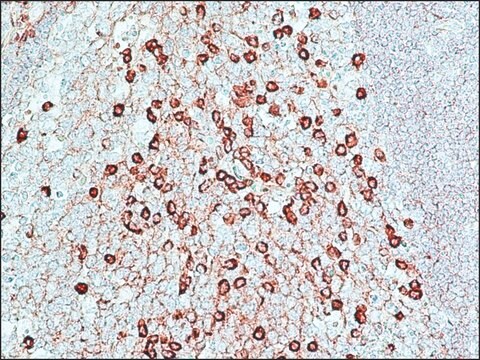
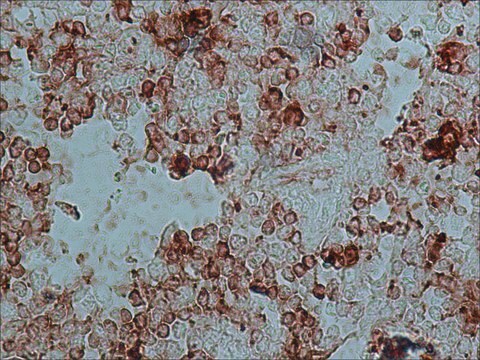
Anti-Rabbit IgG (whole molecule) antibody produced in goat was used for immunohistochemistry of rat brain sections. It was also used in protein spotting assays.
Biochem/physiol Actions
IgG is secreted by B cells and is found in blood and extracellular fluids and provides protection from infections caused by bacteria, fungi and viruses. Maternal IgG is transferred to fetus through the placenta that is vital for immune defense of the neonate against infections.
Preparation Note
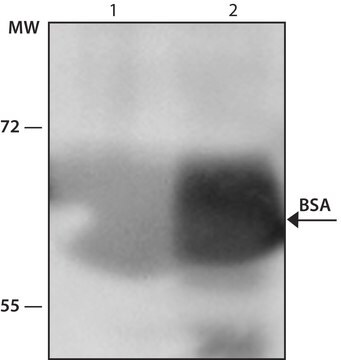
treated to remove lipoproteins
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jian Han et al.
The Journal of nutrition, 132(9), 2769-2774 (2002-09-11)
The mRNA expression of ferritin subunits has not been studied thoroughly in the brain regions of iron-deficient rats. Sprague-Dawley rats (n = 26; 21 d old) were randomly assigned to an iron-deficient (3.5 mg Fe/kg diet) or a control diet
Antonio Porro et al.
Molecular and cellular biology, 30(20), 4808-4817 (2010-08-18)
Telomeres are transcribed into telomeric repeat-containing RNA (TERRA), large, heterogeneous, noncoding transcripts which form part of the telomeric heterochromatin. Despite a large number of functions that have been ascribed to TERRA, little is known about its biogenesis. Here, we present
Jeongsik Yong et al.
The EMBO journal, 21(5), 1188-1196 (2002-02-28)
The survival of motor neurons (SMN) protein complex functions in the biogenesis of spliceosomal small nuclear ribonucleoprotein particles (snRNPs) and prob ably other RNPs. All spliceosomal snRNPs have a common core of seven Sm proteins. To mediate the assembly of
Emre Ozkumur et al.
Proceedings of the National Academy of Sciences of the United States of America, 105(23), 7988-7992 (2008-06-05)
Direct monitoring of primary molecular-binding interactions without the need for secondary reactants would markedly simplify and expand applications of high-throughput label-free detection methods. A simple interferometric technique is presented that monitors the optical phase difference resulting from accumulated biomolecular mass.
Chulmin Joo et al.
Biosensors & bioelectronics, 25(2), 275-281 (2009-08-14)
Quantitative measurement of affinities and kinetics of various biomolecular interactions such as protein-protein, protein-DNA and receptor-ligand is central to our understanding of basic molecular and cellular functions and is useful for therapeutic evaluation. Here, we describe a laser-scanning quantitative imaging
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service