HPR116080
Differentiated HepaRG™ Cells Cryopreserved
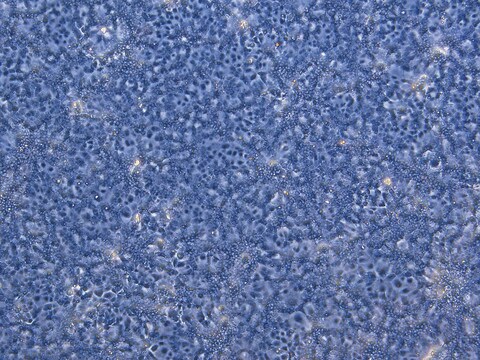
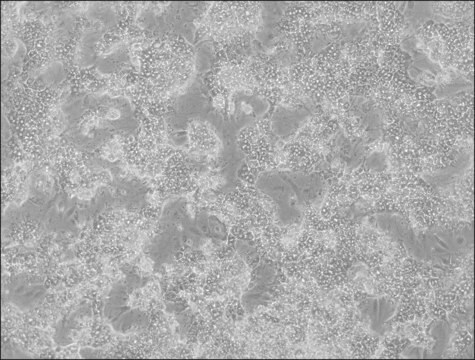
Liver tumor of female patient diagnosed with hepatocarcinoma and hepatitis
Synonym(s):
HepaRG Cell Line
About This Item
product name
Differentiated HepaRG™ Cells Cryopreserved,
biological source
(Liver tumor of female patient diagnosed with hepatocarcinoma and hepatitis C)
shipped in
dry ice
storage temp.
−196°C
General description
Application
Features and Benefits
Legal Information
HepaRG cells are patented and their use is strictly limited; consider the cells as a single use, disposable product that must be destroyed upon conclusion of a study or experiment. Propagating, reproducing, cloning, subcloning or any other use of the cells following the conclusion of a study is prohibited. Use of the cells to produce or manufacture commercial products for general sale or for use in the manufacture of products intended for general sale is prohibited. Transfer of the cells to anyone not employed within the same organization, whether for financial benefit or not, is prohibited. If you are unwilling to accept the terms of this LIMITED USE LICENSE, do not ORDER or use them, and immediately return the cells for credit. Violators of this Limited Use License will be prosecuted to the fullest extent of the law.
Disclaimer
supplement
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Repr. 1A
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Oral drug delivery involves dissolution in the small intestine and absorption across the enterocyte barrier into the portal vein followed by subsequent delivery through the liver into the systemic circulation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service