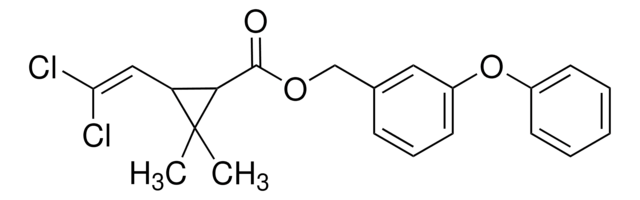
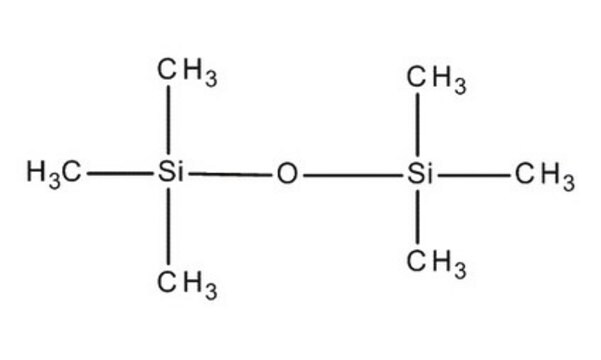
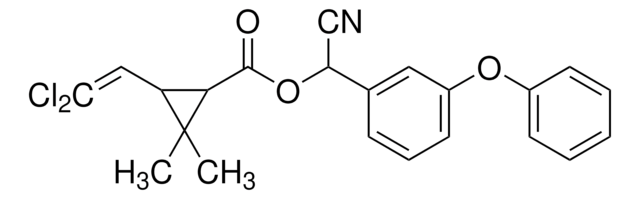
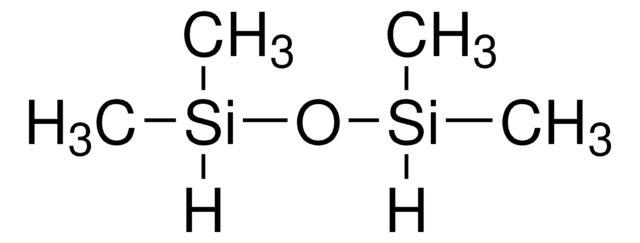
52630
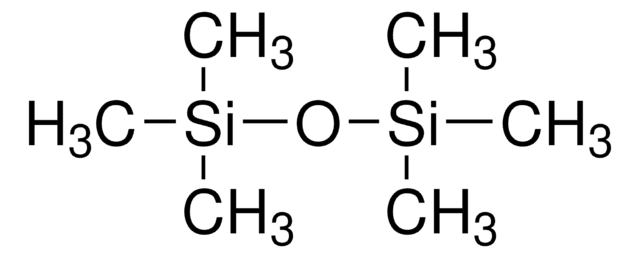
Hexamethyldisiloxane
puriss., ≥98.5% (GC)
Synonym(s):
HMDSO
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)3SiOSi(CH3)3
CAS Number:
Molecular Weight:
162.38
Beilstein:
1736258
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
>1 (vs air)
Quality Level
grade
puriss.
Assay
≥98.5% (GC)
form
liquid
refractive index
n20/D 1.377 (lit.)
n20/D 1.377
bp
101 °C (lit.)
mp
−59 °C (lit.)
density
0.764 g/mL at 20 °C (lit.)
SMILES string
C[Si](C)(C)O[Si](C)(C)C
InChI
1S/C6H18OSi2/c1-8(2,3)7-9(4,5)6/h1-6H3
InChI key
UQEAIHBTYFGYIE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
HMDSO(hexamethyldisiloxane) is an organosilicon compound. It is a non-toxic, environmentally friendly monomer. It can be used as a precursor to synthesize organosilicon thin films. It serves as a silylating agent for carboxylic acids and alcohols. Additionally, it is used in the preparation of aroyl chlorides. HMDSO is commonly used as a water-repellent.
Application
Hexamethyldisiloxane is used:
- As a modifying agent in the presence of ethanol/n-hexane solution for the preparation of hydrophobic and hydrophilic sodium silicate-based silica xerogels.
- To form the dielectric layers in the MIM(metal-insulator-metal) structure that is consisting of graphene quantum dots(GQDs) to prepare two-terminal NVM (non-volatile-memory) devices.
Features and Benefits
Hexamethyldisiloxanehas high thermal stability.
Other Notes
For a facile silylation of carboxylic acids; As chloride anion acceptor in the synthesis of sulphinic esters
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
21.2 °F - closed cup
Flash Point(C)
-6 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
H. Matsumoto et al.
Chemistry Letters (Jpn), 1475-1475 (1980)
J. Drabowicz
Chemistry Letters (Jpn), 1753-1753 (1981)
J. Heberle et al.
Silylating Agents, 2nd ed. (1995)
Pavel Solař et al.
Scientific reports, 7(1), 8514-8514 (2017-08-19)
Nanoparticles composed of multiple silver cores and a plasma polymer shell (multicore@shell) were prepared in a single step with a gas aggregation cluster source operating with Ar/hexamethyldisiloxane mixtures and optionally oxygen. The size distribution of the metal inclusions as well
Anastasia Spyrogianni et al.
Journal of colloid and interface science, 507, 95-106 (2017-08-07)
The surface chemistry of synthetic amorphous silicas is essential for their applicational performance and for understanding their interactions with biological matter. Synthesis of silica by flame spray pyrolysis (FSP) allows to control the content and type of hydroxyl groups which
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service