324355
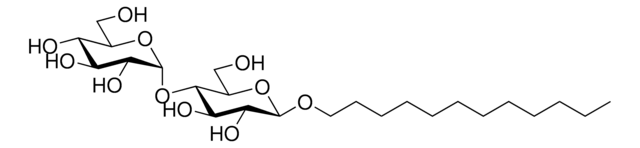
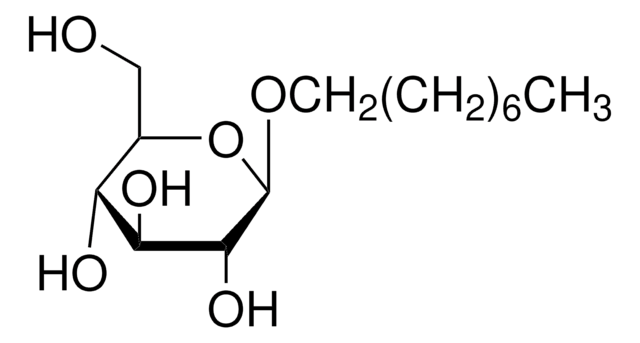
n-Dodecyl β-D-maltoside
ULTROL® Grade, ≥98% (HPLC), non-ionic detergent for the stabilization and activation of enzymes
Synonym(s):
n-Dodecyl-β-D-maltoside, ULTROL® Grade, DDM, Dodecyl maltoside, Lauryl-β-D-maltoside
About This Item
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
solid
mol wt
micellar wt 50000
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
desiccated (hygroscopic)
aggregation number
98
impurities
≤1% dodecanol 1H NMR
color
white
CMC
100-600 μM
solubility
water: 100 mg/mL
shipped in
ambient
storage temp.
2-8°C
InChI
1S/C24H46O11/c1-2-3-4-5-6-7-8-9-10-11-12-32-23-21(31)19(29)22(16(14-26)34-23)35-24-20(30)18(28)17(27)15(13-25)33-24/h15-31H,2-14H2,1H3/t15-,16-,17-,18+,19-,20-,21-,22-,23-,24-/m1/s1
InChI key
NLEBIOOXCVAHBD-QKMCSOCLSA-N
General description
Application
- Maximizing hydrophobic peptide recovery in proteomics and antibody development using a mass spectrometry compatible surfactant.: The article explores the use of n-Dodecyl β-D-maltoside to maximize hydrophobic peptide recovery in proteomics. The findings highlight the surfactant′s compatibility with mass spectrometry, improving peptide analysis and antibody development. (Nie et al., 2022).
- Sickle-like Inertial Microfluidic System for Online Rare Cell Separation and Tandem Label-Free Quantitative Proteomics (Orcs-Proteomics).: This research introduces an inertial microfluidic system utilizing n-Dodecyl β-D-maltoside for rare cell separation and proteomics. The study underscores the surfactant′s role in enhancing cell recovery and proteomic analysis. (Wang et al., 2022).
- Surfactant-assisted one-pot sample preparation for label-free single-cell proteomics.: This paper presents a method for single-cell proteomics using n-Dodecyl β-D-maltoside. The surfactant assists in efficient sample preparation, facilitating high-resolution proteomic analysis. (Tsai et al., 2021).
Warning
Reconstitution
Stock solutions are stable for up to 3 months at 4°C.
Other Notes
Bass, W.T., and Bricker, T.M. 1988. Anal. Biochem.171, 330.
Rosevear, P., et al. 1980. Biochemistry19, 4108.
Grip, W.J., and Bovee-Geurts, P.H.M. 1979. Chem. Phys. Lipids23, 321.
Stubbs, G.W., et al. 1976. Biochim. Biophys. Acta425, 46.
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service