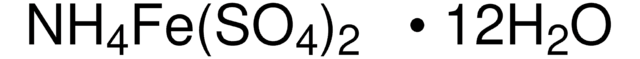1.09864
Ammonium iron(II) sulfate solution
c[(NH₄)₂ Fe(SO₄)₂] = 0.1 mol/l (0.1 N), concentrate for 250 mL titration solution, Titrisol®
Synonym(s):
Ammonium Ferrous Sulfate Solution
About This Item
Recommended Products
product name
Ammonium iron(II) sulfate solution, for 250 mlc[(NH₄)₂ Fe(SO₄)₂] = 0.1 mol/l (0.1 N) Titrisol®
Quality Level
product line
Titrisol®
form
liquid
reaction suitability
reaction type: Complexometric reactions
concentration
0.1 M
technique(s)
titration: suitable
pH
0.4 (20 °C in H2O)
density
1.25 g/cm3 at 20 °C
storage temp.
15-25°C
Application
- Regulation of Iron-Ion Transporter SLC11A2 by Three Identical miRNAs.: This study investigates the modulation of iron ion transporters, providing insights into molecular processes involving ammonium iron(II) sulfate, crucial for analytical chemistry applications (Sugino et al., 2022).
- Two-Dimensional Bis(dithiolene)iron(II) Self-Powered UV Photodetectors with Ultrahigh Air Stability.: This research highlights the development of robust UV photodetectors using iron(II)-based materials, demonstrating the potential of ammonium iron(II) sulfate in creating high-performance electronic devices (Wang YC et al., 2021).
- Pencil it in: pencil drawn electrochemical sensing platforms.: Focuses on innovative, cost-effective electrochemical sensors where iron plays a critical role, pertinent to applications in chemical analysis (Foster CW et al., 2016).
- Catalytic effect of different forms of iron in purification of single-walled carbon nanotubes.: Discusses the use of various iron forms, including ammonium iron(II) sulfate, in enhancing the purification processes for carbon-based materials, significant in analytical applications (Suzuki T et al., 2010).
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1A
Storage Class Code
8B - Non-combustible, corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Protocols
Polymers are hardly or not at all soluble in the common Karl Fischer solvents. In order to extract the water trapped within the sample the plastic must be dissolved or swollen by means of an appropriate solvent to such an extent that the water can be released through diffusion. Which solvent is appropriate depends on the properties of the plastic.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service