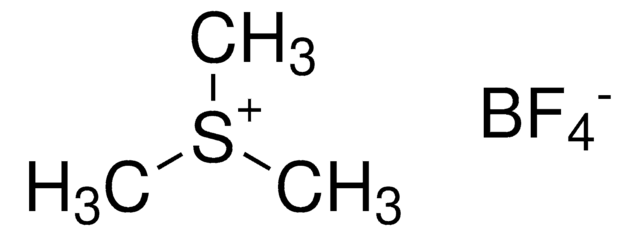
T80489
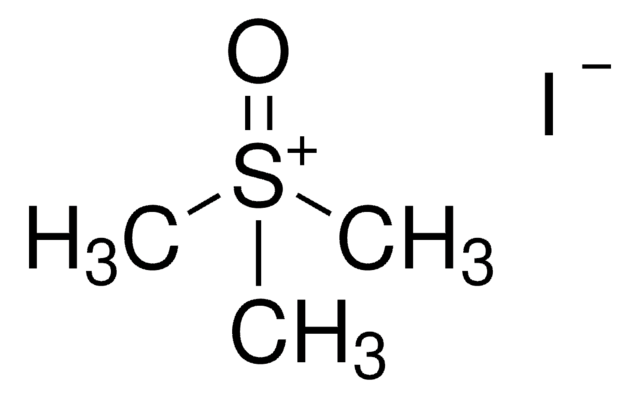
Trimethylsulfonium iodide
98%
Synonym(s):
Trimethyl-λ[3]-sulfane hydroiodide, Trimethylsulphonium iodide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(CH3)3S(I)
CAS Number:
Molecular Weight:
204.07
Beilstein:
3555192
EC Number:
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
reaction suitability
reaction type: C-C Bond Formation
mp
215-220 °C (lit.)
SMILES string
[I-].C[S+](C)C
InChI
1S/C3H9S.HI/c1-4(2)3;/h1-3H3;1H/q+1;/p-1
InChI key
VFJYIHQDILEQNR-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
Trimethylsulfonium iodide is commonly used as a methylating agent in organic synthesis.
Application
Treatment with strong base yields the dimethylsulfonium methylide which reacts in-situ with the carbonyl group of ketones to form epoxides or allylic alcohols.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C Díez et al.
Journal of chromatography. A, 1125(2), 244-253 (2006-06-20)
In this study, an orthogonal array design was applied to know the way different parameters affected the derivatization of some herbicides that are commonly applied in the soils. Herbicides formulated as esters have been reported to rapidly hydrolyse, in contact
Julien Dron et al.
Journal of chromatography. A, 1047(1), 111-116 (2004-10-16)
A procedure for the determination of fatty acids (FA) and glycerol in oils has been developed. The method includes a derivatization step of the FAs into their methyl esters or a transesterification of the triacylglycerols with trimethylsulfonium hydroxide (TMSH), respectively.
J L Hoffman
Journal of chromatography, 588(1-2), 211-216 (1991-12-27)
The use of single-column ion chromatography with conductometric detection was shown to be useful for the analysis of sulfonium and selenonium ions. A Hamilton PRP X-200 cation column was eluted with either solvent A (5 mM nitric acid in 30%
C M Kerchove et al.
Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas, 35(4), 485-491 (2002-04-18)
Trimethylsulfonium, a compound present in the midgut gland of the sea hare Aplysia brasiliana, negatively modulates vagal response, indicating a probable ability to inhibit cholinergic responses. In the present study, the pharmacological profile of trimethylsulfonium was characterized on muscarinic and
Lawrence Akoto et al.
Journal of chromatography. A, 1186(1-2), 365-371 (2007-09-25)
Gas chromatography (GC) has in recent times become an important tool for the fatty acid profiling of human blood and plasma. An at-line procedure used in the fatty acid profiling of whole/intact aquatic micro-organisms without any sample preparation was adapted
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service