T71501
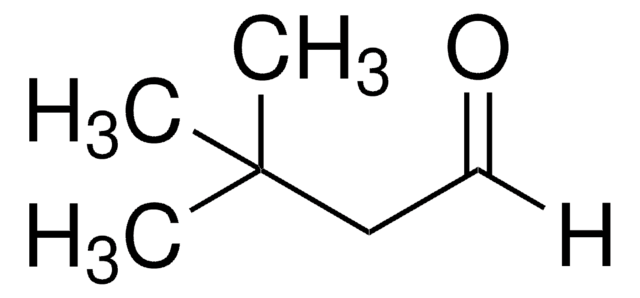
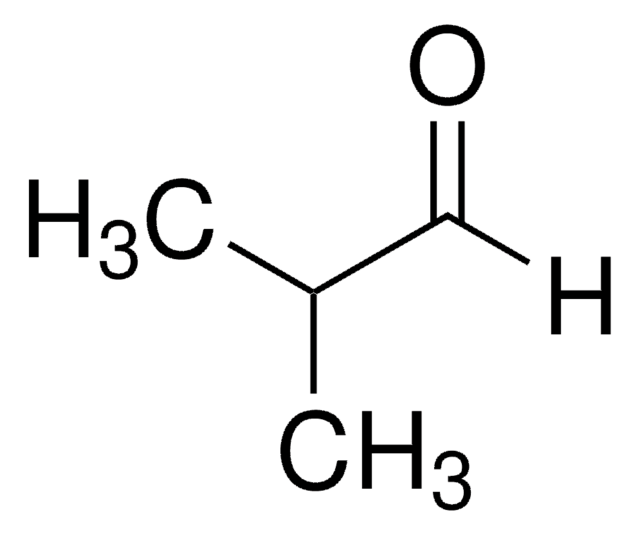
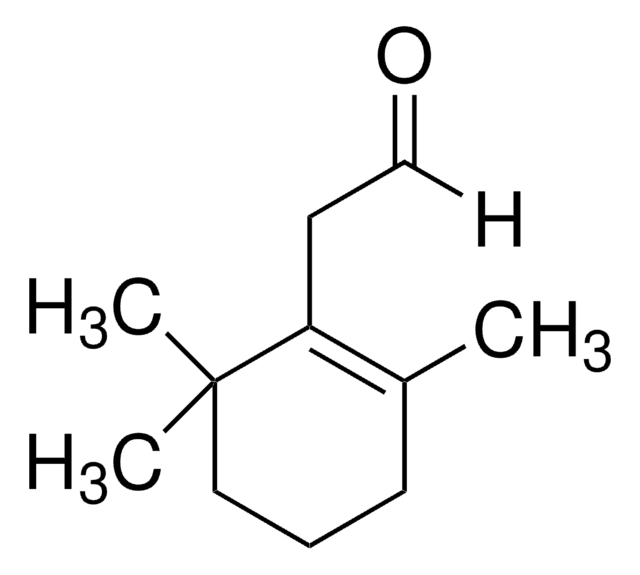
Trimethylacetaldehyde
96%
Synonym(s):
Pivalaldehyde, Trimethylacetaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)3CCHO
CAS Number:
Molecular Weight:
86.13
Beilstein:
506060
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
refractive index
n20/D 1.378 (lit.)
bp
74 °C/730 mmHg (lit.)
mp
6 °C (lit.)
density
0.793 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
[H]C(=O)C(C)(C)C
InChI
1S/C5H10O/c1-5(2,3)4-6/h4H,1-3H3
InChI key
FJJYHTVHBVXEEQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Commonly used building block in aldol condensation reactions.
Employed in a study of the asymmetric cyanation of aldehydes with TMS-CN and a chiral vandium (V)-salen complex.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
3.2 °F - closed cup
Flash Point(C)
-16 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Harry W Gibson et al.
The Journal of organic chemistry, 72(15), 5759-5770 (2007-06-27)
Chiral acid chlorides were reacted with isoquinoline and 6,7-dimethoxy-3,4-dihydroisoquinoline to form diastereomeric Reissert compounds 8-11 and 18-21, respectively. The best diastereoselectivity (80:20) was achieved in formation of the 9-phenylmenthyl derivative 20. The diastereomers of 2-l-menthoxycarbonyl-1,2-dihydroisoquinaldonitriles (S)-8/(R)-8), formed in equal amounts
Li Huang et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(17), 5302-5313 (2012-03-22)
The catalytic asymmetric aziridination of imines and diazo compounds (AZ reaction) mediated by boroxinate catalysts derived from the VANOL and VAPOL ligands was investigated with chiral imines derived from five different chiral, disubstituted, methyl amines. The strongest matched and mismatched
Tetrahedron Asymmetry, 17, 2659-2659 (2006)
Adrian Méndez et al.
Molecules (Basel, Switzerland), 25(21) (2020-11-04)
Singlet oxygen ene reactions produce 2-(tert-butyl)-4a-hydroperoxy-3-methyl-2,4a, 5,6,7,8-hexahydroquinazolin-4(3H)-one quantitatively during diffusion crystallization of 2-(tert-butyl)-3-methyl-2,3,5,6,7,8-hexahydroquinazolin-4(1H)-one in n-hexane/CH2Cl2 solvent mixture. To confirm this photo-oxidation, a 1H-NMR study in CDCl3 was performed with exposure to ambient conditions (light and oxygen), with neither additional reactants
Pierre Awad et al.
Journal of agricultural and food chemistry, 65(35), 7736-7748 (2017-08-02)
Cognac wine spirit has a complex composition in volatile compounds which contributes to its organoleptic profile. This work focused on the batch distillation process and, in particular, on volatile compounds specifically produced by chemical reactions during the distillation of Cognac
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service