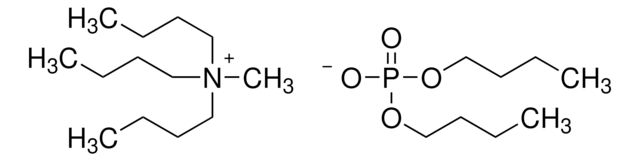
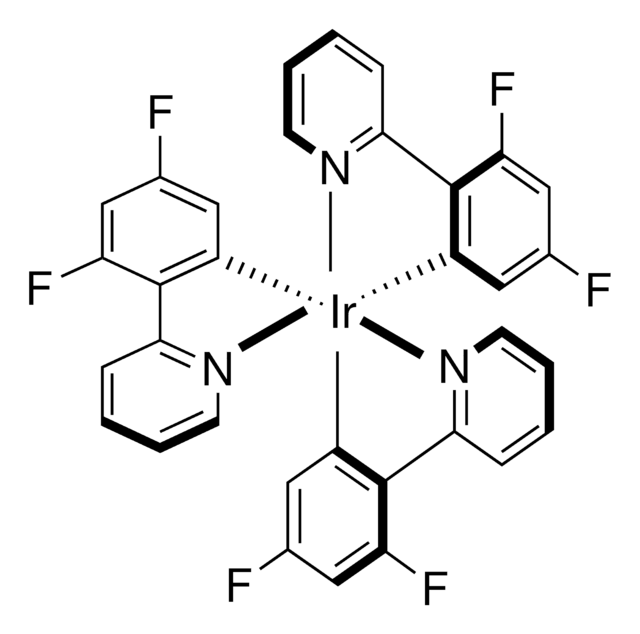
908568
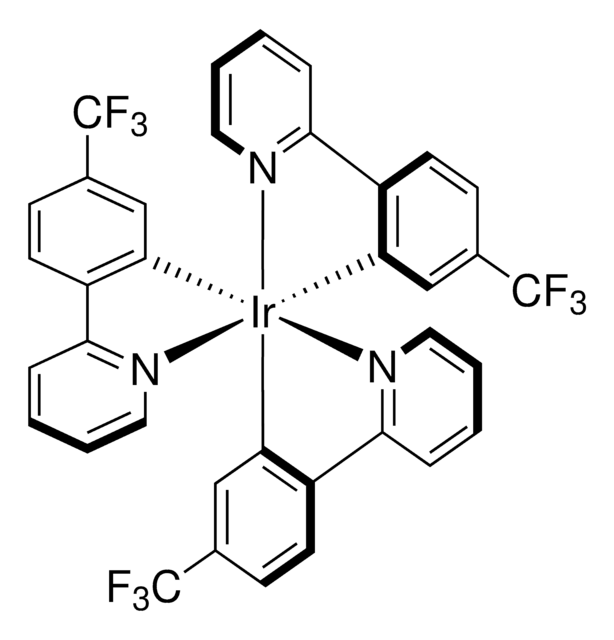
[Ir(dFCF3ppy)2-(5,5′-dCF3bpy)]PF6
≥95%
Synonym(s):
[5,5′-Bis(trifluoromethyl)-2,2′-bipyridine-N1,N1′]bis[3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl-N]phenyl-C]Iridium(III) hexafluorophosphate
About This Item
Recommended Products
Assay
≥95%
form
powder or crystals
reaction suitability
reaction type: Photocatalysis
reagent type: catalyst
mp
>300 °C
photocatalyst activation
460 nm
SMILES string
[Ir+](c5c(c(cc(c5)F)F)c6ncc(cc6)C(F)(F)F)c3c(c(cc(c3)F)F)c4ncc(cc4)C(F)(F)F.FC(F)(F)c1cnc(cc1)c2ncc(cc2)C(F)(F)F.F[P-](F)(F)(F)(F)F
InChI
1S/C12H6F6N2.2C12H5F5N.F6P.Ir/c13-11(14,15)7-1-3-9(19-5-7)10-4-2-8(6-20-10)12(16,17)18;2*13-8-2-3-9(10(14)5-8)11-4-1-7(6-18-11)12(15,16)17;1-7(2,3,4,5)6;/h1-6H;2*1-2,4-6H;;/q;;;-1;+1
InChI key
QEXYETWHTSSWGM-UHFFFAOYSA-N
Related Categories
Application
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
An organic reaction toolbox provides structure-activity relationships for unique chemical reactions to optimally design and control small molecule synthesis.
Photoredox catalysis utilizes photocatalysts in the presence of visible light to form an array of synthetic transformations including, but not limited to, cross-coupling, C-H functionalization, alkene and arene functionalization, and trifluoromethylation.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 908568-100MG | |
| 908568-500MG |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)
![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)
![[Ir{dFCF3ppy}2(bpy)]PF6](/deepweb/assets/sigmaaldrich/product/structures/180/924/79119ac4-7d62-429d-b23d-a14c012c6050/640/79119ac4-7d62-429d-b23d-a14c012c6050.png)
![[Ir(dF(Me)ppy)2(dtbbpy)]PF6](/deepweb/assets/sigmaaldrich/product/structures/150/099/7c2dfa31-39f4-4cca-aee5-86d4a89fea78/640/7c2dfa31-39f4-4cca-aee5-86d4a89fea78.png)
![Ir[dFFppy]2-(4,4′-dCF3bpy)PF6 ≥95%](/deepweb/assets/sigmaaldrich/product/structures/816/772/b116c17c-e6b2-4c95-be64-45a5a851d823/640/b116c17c-e6b2-4c95-be64-45a5a851d823.png)
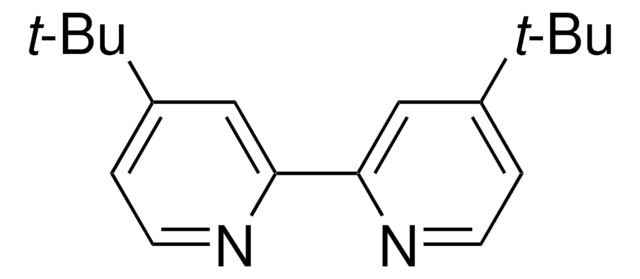
![[Ir(dFppy)2(dtbbpy)]PF6](/deepweb/assets/sigmaaldrich/product/structures/258/715/c8fe85d5-be71-4ff1-849b-a20766636770/640/c8fe85d5-be71-4ff1-849b-a20766636770.png)
![Ir[dFMeppy]2-(4,4′-dCF3bpy)PF6 ≥95%](/deepweb/assets/sigmaaldrich/product/structures/355/743/1aef4eef-372d-4f1d-8904-08df7c4fd417/640/1aef4eef-372d-4f1d-8904-08df7c4fd417.png)


![[Ru(bpz)3][PF6]2 95%](/deepweb/assets/sigmaaldrich/product/structures/317/925/f0ef928e-bbea-4535-abe6-dda0bc28d32a/640/f0ef928e-bbea-4535-abe6-dda0bc28d32a.png)
![Ir[dF(t-Bu)-ppy]3](/deepweb/assets/sigmaaldrich/product/structures/254/294/d0fb19e5-05b2-4c1b-990b-a99fa60b3e73/640/d0fb19e5-05b2-4c1b-990b-a99fa60b3e73.png)
![Ir[p-F(t-Bu)-ppy]3](/deepweb/assets/sigmaaldrich/product/structures/189/186/7badaac3-82af-4109-aab5-dea3a3aa916d/640/7badaac3-82af-4109-aab5-dea3a3aa916d.png)