904937
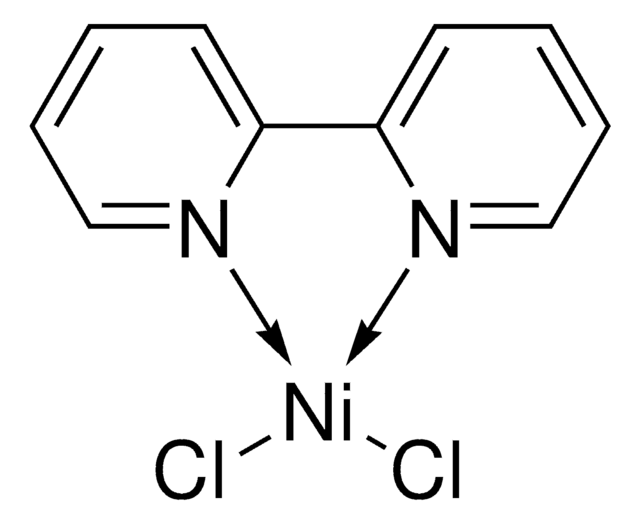
2,6-Bis(N-pyrazolyl)pyridine nickel (II) dichloride
≥95% anhydrous basis
Synonym(s):
(bpp)NiCl2
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C11H9Cl2N5Ni
CAS Number:
Molecular Weight:
340.82
MDL number:
UNSPSC Code:
12352101
NACRES:
NA.22
Recommended Products
Assay
≥95% anhydrous basis
form
powder or crystals
reaction suitability
core: nickel
reaction type: Cross Couplings
reagent type: catalyst
mp
107.9-132.4 °C (Decomp)
Application
2,6-Bis(N-pyrazolyl)pyridine nickel (II) dichloride ((bpp)NiCl2) is a Ni precatalyst that can be used in Negishi alkyl-alkyl cross-coupling, reductive cross-coupling of styrenyl aziridines, and dialkyl ether formation.
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Brian P Woods et al.
Journal of the American Chemical Society, 139(16), 5688-5691 (2017-04-14)
A Ni-catalyzed reductive cross-coupling of styrenyl aziridines with aryl iodides is reported. This reaction proceeds by a stereoconvergent mechanism and is thus amenable to asymmetric catalysis using a chiral bioxazoline ligand for Ni. The process allows facile access to highly
Dialkyl Ether Formation by Nickel-Catalyzed Cross-Coupling of Acetals and Aryl Iodides.
Arendt KM and Doyle AG
Angewandte Chemie (International Edition in English), 54(34), 9876-9880 (2015)
Nickel-Catalyzed Negishi Cross-Couplings of Secondary Nucleophiles with Secondary Propargylic Electrophiles at Room Temperature.
Smith SW and Fu GC
Angewandte Chemie (International ed. in English), 47(48), 9334-9336 (2008)
Nickel-catalyzed enantioselective reductive cross-coupling of styrenyl aziridines.
Woods BP, et al.
Journal of the American Chemical Society, 139(16), 5688-5691 (2017)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![[4,4′-Bis(1,1-dimethylethyl)-2,2′-bipyridine] nickel (II) dichloride](/deepweb/assets/sigmaaldrich/product/structures/471/091/6faa29b1-bf8a-4d87-90b2-4cc55e082620/640/6faa29b1-bf8a-4d87-90b2-4cc55e082620.png)



![[(TMEDA)Ni(o-tolyl)Cl] 95%](/deepweb/assets/sigmaaldrich/product/structures/236/439/768c916e-994f-47e3-a980-3ca0471317d7/640/768c916e-994f-47e3-a980-3ca0471317d7.png)
![[1,3-Bis(diphenylphosphino)propane]dichloronickel(II)](/deepweb/assets/sigmaaldrich/product/structures/844/065/af07f787-c6a3-4a6e-a22b-47a933c73978/640/af07f787-c6a3-4a6e-a22b-47a933c73978.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloronickel(II) 97%](/deepweb/assets/sigmaaldrich/product/structures/274/566/a60d6584-163a-4c41-a738-60f8e4d524fa/640/a60d6584-163a-4c41-a738-60f8e4d524fa.png)