807664
CBTF
Synonym(s):
APN-TFS ester, Sodium 4-((4-(cyanoethynyl)benzoyl)oxy)-2,3,5,6-tetrafluorobenzenesulfonate
About This Item
Recommended Products
form
powder
Quality Level
reaction suitability
reagent type: cross-linking reagent
functional group
ester
storage temp.
−20°C
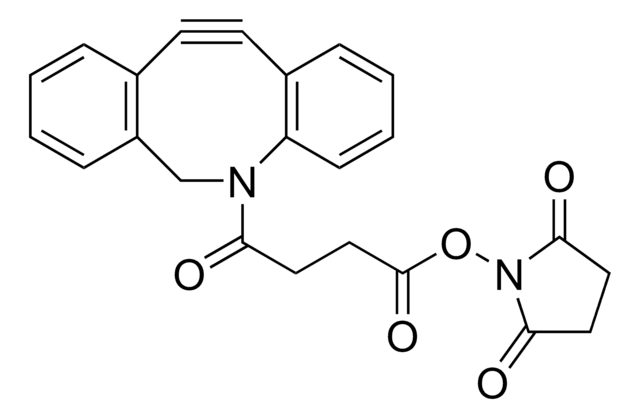
SMILES string
O=C(OC1=C(F)C(F)=C(S(=O)(O[Na])=O)C(F)=C1F)C2=CC=C(C#CC#N)C=C2
InChI
1S/C16H5F4NO5S.Na/c17-10-12(19)15(27(23,24)25)13(20)11(18)14(10)26-16(22)9-5-3-8(4-6-9)2-1-7-21;/h3-6H,(H,23,24,25);/q;+1/p-1
InChI key
YFJYSJRZDOWXDH-UHFFFAOYSA-M
General description
Application
Other Notes
- Dissolve the protein in an appropriate buffer* with pH 7.5-9.0 (e.g. PBS) at 1-10mg/mL concentration.
- Apply the appropriate amount of the stock solution of the reagent (1-5 molar eq. per lysine residue).
- Incubate at room temperature for 2 hours.
- If necessary, purify the protein-APN conjugate using size exclusion chromatography or ultrafiltration.
- The conjugate can be readily coupled with thiol-containing substrates by incubating the components in aqueous buffer (pH 6.5-9.0) at ambient temperature for 2 hours.
Standard protein labeling procedure (cysteine labeling):
Dissolve the protein in the appropriate buffer* with pH 6.5-9.0 (e.g. PBS) at 1-10mg/mL concentration.
Apply the appropriate amount of the stock solution of the APN-labeled molecule (1-5 molar eq. per free cysteine residue).
Incubate at room temperature for 2 hours.
If necessary, purify the protein conjugate using size exclusion chromatography or ultrafiltration.
*Note: avoid amine- and thiol-containing buffers.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![LC-SPDP (succinimidyl 6-[3(2-pyridyldithio)propionamido]hexanoate)](/deepweb/assets/sigmaaldrich/product/structures/300/586/d95fd80c-e201-4b0b-8aee-31e109c2ff41/640/d95fd80c-e201-4b0b-8aee-31e109c2ff41.png)






![(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethyl N-succinimidyl carbonate for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/969/022/d6776082-2f7a-47c7-bcd4-3830dac0fb7d/640/d6776082-2f7a-47c7-bcd4-3830dac0fb7d.png)