777692
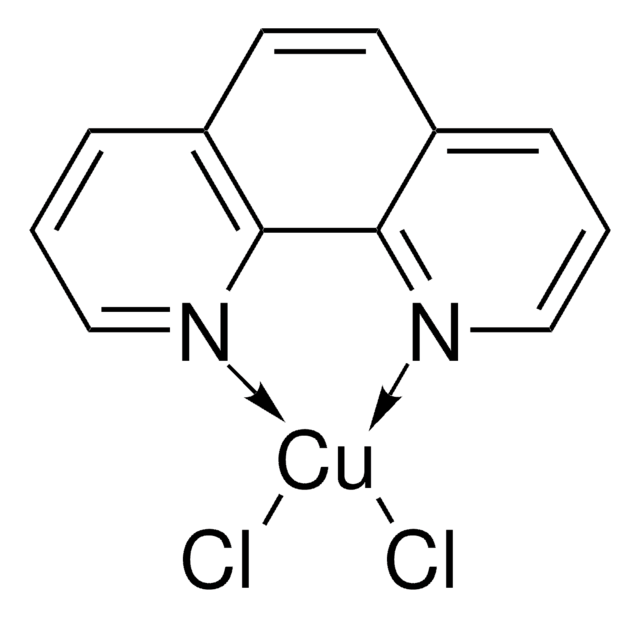
(1,10-Phenanthroline)(trifluoromethyl)copper(I)
90%
Synonym(s):
Trifluoromethylator®
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C13H8CuF3N2
CAS Number:
Molecular Weight:
312.76
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
90%
form
solid
storage temp.
2-8°C
InChI
1S/C12H8N2.CF3.Cu/c1-3-9-5-6-10-4-2-8-14-12(10)11(9)13-7-1;2-1(3)4;/h1-8H;;
InChI key
CUQZQRIIFNWQSJ-UHFFFAOYSA-N
General description
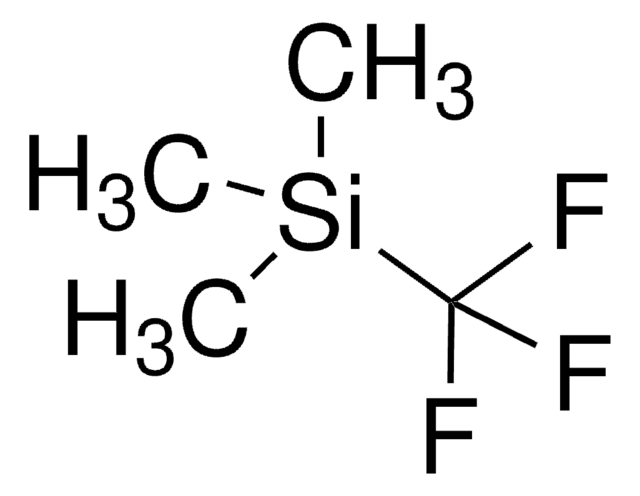
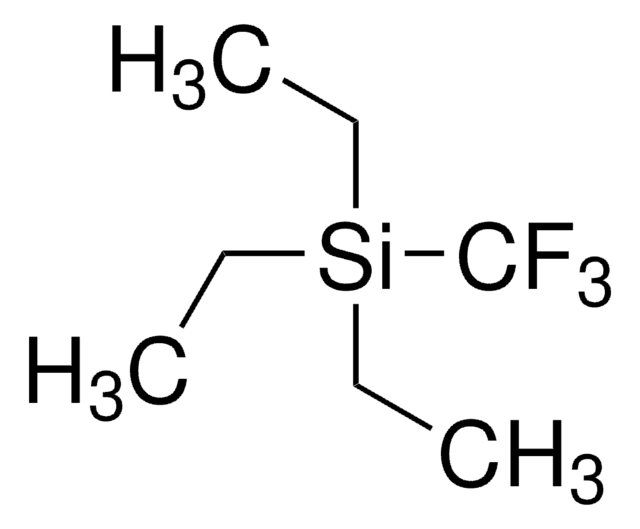
(1,10-Phenanthroline)(trifluoromethyl)copper(I) [(Phen)Cu-CF3] can be prepared by treating [CuOt-Bu]4 with 1,10-phenanthroline, followed by the addition of (trifluoromethyl)trimethylsilane (CF3TMS). It is an easily handled, thermally stable, single-component reagent.
Application
Trifluoromethylator, A New Arene Trifluoromethylation Reagent: (Phen)Cu-CF3
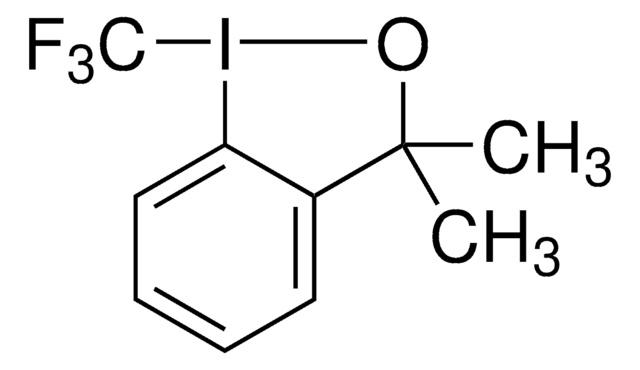
(Phen)Cu-CF3 can be used as a reagent for the trifluoromethylation of aryl iodides. Electron-rich and electron-deficient iodoarenes, as well as sterically-hindered iodoarenes, react in high yield under mild conditions. Electrophilic functional groups, including aldehydes, nitroarenes, ketones, and esters are tolerated. The substrate scope of this reagent is broad and the reagent has wide applicability for trifluoromethylation reactions.
It can also be used in the oxidative trifluoromethylation of organoboron reagents, and terminal alkynes through C-H activation.
(Phen)Cu-CF3 can be used as a reagent for the trifluoromethylation of aryl iodides. Electron-rich and electron-deficient iodoarenes, as well as sterically-hindered iodoarenes, react in high yield under mild conditions. Electrophilic functional groups, including aldehydes, nitroarenes, ketones, and esters are tolerated. The substrate scope of this reagent is broad and the reagent has wide applicability for trifluoromethylation reactions.
It can also be used in the oxidative trifluoromethylation of organoboron reagents, and terminal alkynes through C-H activation.
Legal Information
Trifluoromethylator is a registered trademark of Catylix, Inc
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A broadly applicable copper reagent for trifluoromethylations and perfluoroalkylations of aryl iodides and bromides.
Hiroyuki Morimoto et al.
Angewandte Chemie (International ed. in English), 50(16), 3793-3798 (2011-03-29)
Related Content
An organic reaction toolbox provides structure-activity relationships for unique chemical reactions to optimally design and control small molecule synthesis.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service