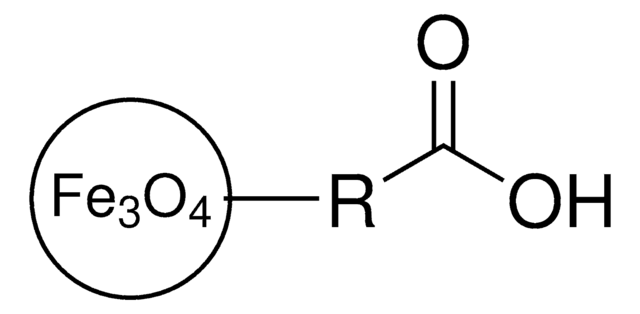
725366
Iron oxide(II,III), magnetic nanoparticles solution
20 nm avg. part. size, 5 mg/mL in H2O
Synonym(s):
Magnetic iron oxide nanocrystals, Magnetite, Superparamagnetic iron oxide nanoparticles
About This Item
Recommended Products
form
dispersion
nanoparticles
Quality Level
concentration
5 mg/mL in H2O
magnetization
>20 emu/g, at 4500Oe
particle size
18-22 nm
avg. part. size
20 nm
density
1.00 g/mL at 25 °C
SMILES string
O=[Fe].O=[Fe]O[Fe]=O
InChI
1S/3Fe.4O
InChI key
SZVJSHCCFOBDDC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Magnetic iron oxide nanoparticle (IONP) synthesis to applications: present and future: This report outlines the co-precipitation synthesis of magnetite nanoparticles using Fe(II) and Fe(III) solutions and discusses their future applications (N Ajinkya et al., 2020).
- Surface modification of magnetic iron oxide nanoparticles: Explores the surface engineering of iron oxide nanoparticles (IONPs) to enhance their functionality for various applications (N Zhu et al., 2018).
- Recent advances on iron oxide magnetic nanoparticles as sorbents of organic pollutants in water and wastewater treatment: Discusses the use of iron oxide magnetic nanoparticles in removing organic pollutants from water, highlighting the synthesis of core-shell magnetic nanoparticles (AM Gutierrez et al., 2017).
- Potential toxicity of iron oxide magnetic nanoparticles: Reviews the potential toxic effects of iron oxide magnetic nanoparticles, emphasizing their stability, biocompatibility, and size control (N Malhotra et al., 2020).
- Co-precipitation in aqueous solution synthesis of magnetite nanoparticles using iron (III) salts as precursors: Details the synthesis process of iron oxide nanocrystals and their potential applications in various fields (MI Khalil, 2015).
Storage Class Code
12 - Non Combustible Liquids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Currently, magnetic nanoparticles (MNPs) are attracting a lot of attention because of the possibility of many novel applications, especially in biomedical research.
A key challenge for nanomaterial safety assessment is the ability to handle the large number of newly engineered nanomaterials (ENMs), including developing cost-effective methods that can be used for hazard screening.
Professor Hui Mao explores the use of superparamagnetic iron oxide nanoparticles (INOPs) that offer an alternate contrast-enhancing mechanism.
Professor Yadong Yin (University of California Riverside, USA) examines both direct (thermal decomposition, solvothermal, hydrothermal) and indirect (templated) synthesis methods of magnetite nanocrystals and reviews in detail the landscape of these various synthetic methods for magnetite nanocrystal and their applications in magnetic assembly, magnetic hyperthermia, and Li-Ion batteries.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 725366-5ML | 4061832861494 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service