718742
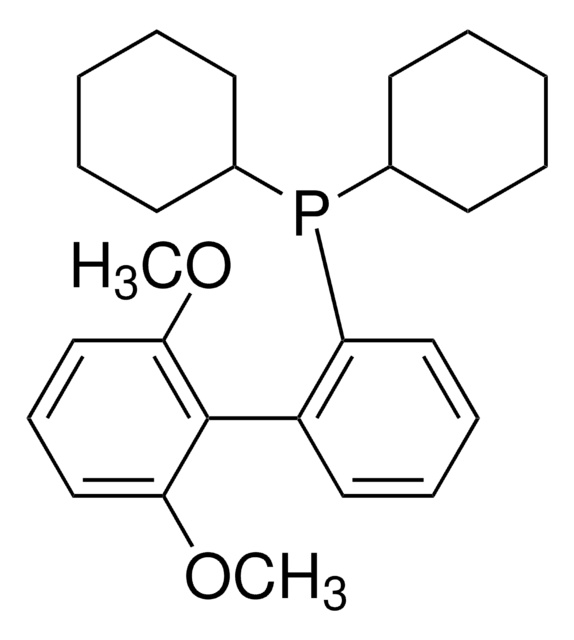
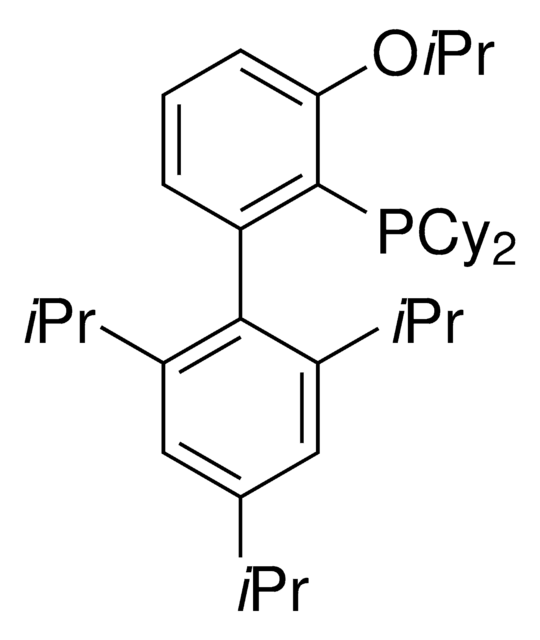
BrettPhos
98%
Synonym(s):
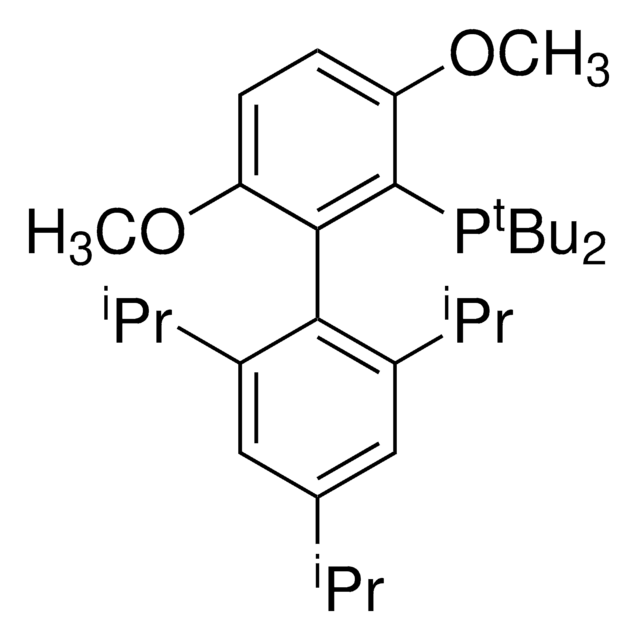
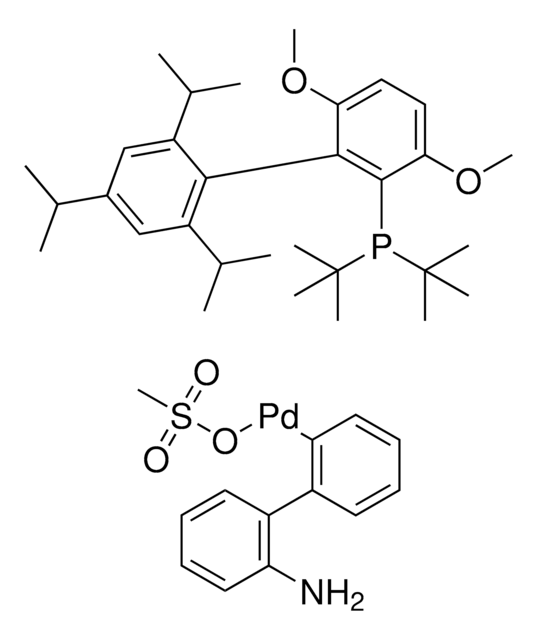
2-(Dicyclohexylphosphino)3,6-dimethoxy-2′,4′,6′-triisopropyl-1,1′-biphenyl
About This Item
Recommended Products
Quality Level
Assay
98%
form
solid
reaction suitability
reaction type: Cross Couplings
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: Fluorinations
greener alternative product score
old score: 8
new score: 1
Find out more about DOZN™ Scoring
greener alternative product characteristics
Waste Prevention
Atom Economy
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
187-195 °C
functional group
phosphine
greener alternative category
SMILES string
COc1c(P(C2CCCCC2)C3CCCCC3)c(c4c(C(C)C)cc(C(C)C)cc4C(C)C)c(OC)cc1
InChI
1S/C35H53O2P/c1-23(2)26-21-29(24(3)4)33(30(22-26)25(5)6)34-31(36-7)19-20-32(37-8)35(34)38(27-15-11-9-12-16-27)28-17-13-10-14-18-28/h19-25,27-28H,9-18H2,1-8H3
InChI key
WDVGNXKCFBOKDF-UHFFFAOYSA-N
General description
Application
It can be used in:
- palladium-catalyzed trifluoromethylation of aryl chlorides
- Buchwald-Hartwig amination
- synthesis of 4-aryl and alkyl substituted, N6-alkylated pyridazine-3,6-diamines via a Buchwald protocol
Features and Benefits
- White crystalline solid
- Air- and moisture-stable
- Thermally stable
- Highly efficient
- Wide functional group tolerance
- Excellent selectivity and conversion
Storage Class Code
11 - Combustible Solids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
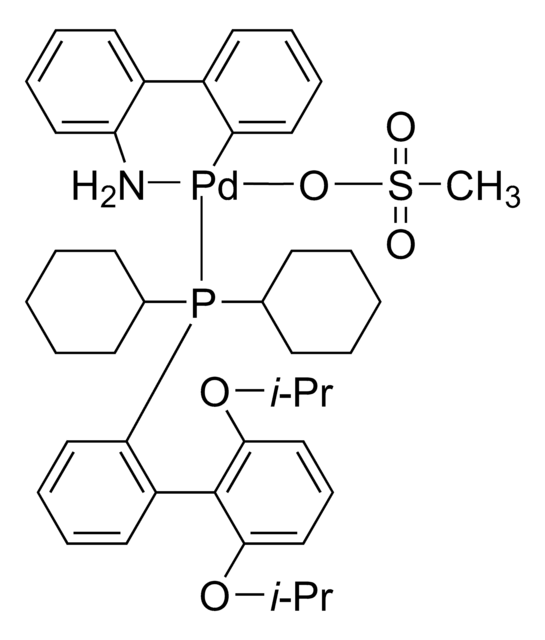
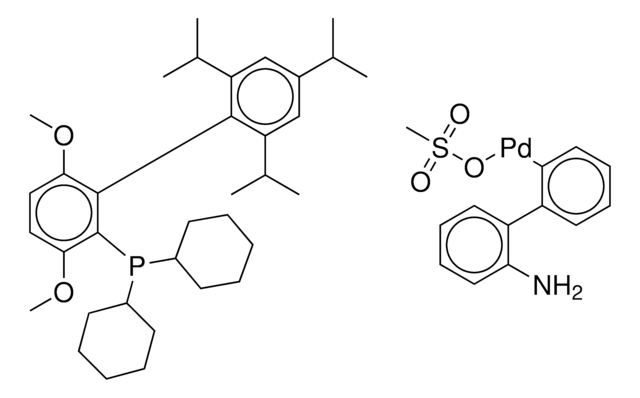
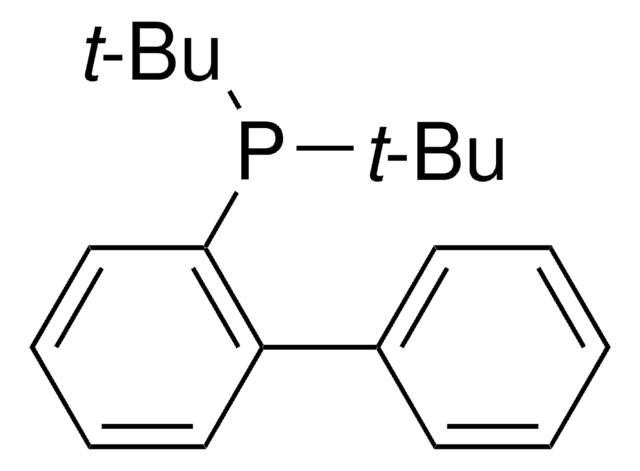
Customers Also Viewed
Related Content
An organic reaction toolbox provides structure-activity relationships for unique chemical reactions to optimally design and control small molecule synthesis.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 718742-25G | 4061832855196 |
| 718742-100MG | 4061833239803 |
| 718742-1G | 4061832855189 |
| 718742-500MG | 4061833239810 |
| 718742-50G | 4061833551479 |
| 718742-5G | 4061832855202 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![[2-(Dicyclohexylphosphino)-3,6-dimethoxy-2′,4′,6′-triisopropyl-1,1′-biphenyl]gold(I) bis(trifluoromethanesulfonyl)imide](/deepweb/assets/sigmaaldrich/product/structures/361/949/e30e9505-889a-4ffd-9c57-f66a0a20b299/640/e30e9505-889a-4ffd-9c57-f66a0a20b299.png)