669032
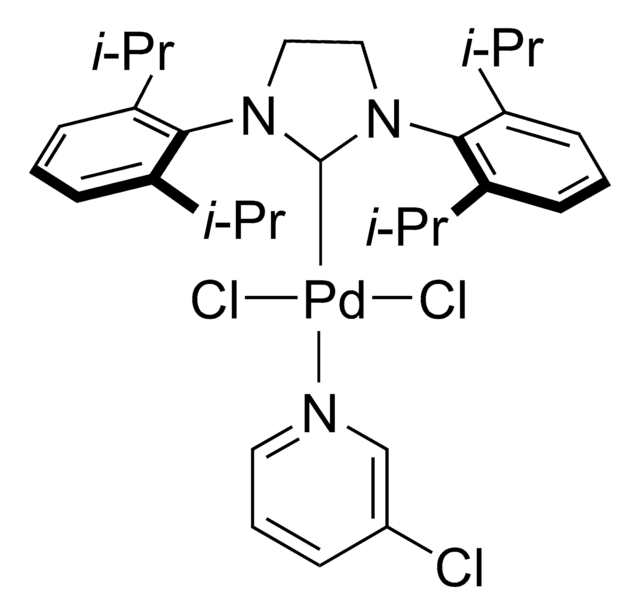
PEPPSI™-IPr catalyst
98%, Umicore
Synonym(s):
[1,3-Bis(2,6-Diisopropylphenyl)imidazol-2-ylidene](3-chloropyridyl)palladium(II) dichloride
About This Item
Recommended Products
Quality Level
Assay
98%
reaction suitability
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
manufacturer/tradename
Umicore
mp
230 °C
SMILES string
Cl[Pd]Cl.Clc1cccnc1.CC(C)c2cccc(C(C)C)c2N3CN(C=C3)c4c(cccc4C(C)C)C(C)C
InChI
1S/C27H38N2.C5H4ClN.2ClH.Pd/c1-18(2)22-11-9-12-23(19(3)4)26(22)28-15-16-29(17-28)27-24(20(5)6)13-10-14-25(27)21(7)8;6-5-2-1-3-7-4-5;;;/h9-16,18-21H,17H2,1-8H3;1-4H;2*1H;/q;;;;+2/p-2
InChI key
BLDKGTGQENJFON-UHFFFAOYSA-L
Related Categories
General description
Application
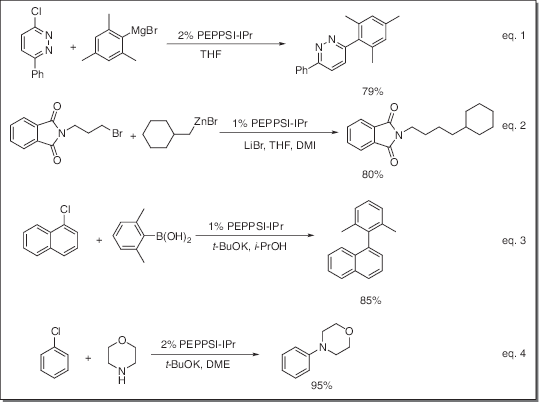
- Catalyst for Kumada-Tamao-Corriu (KTC) reaction (eq. 1)
- Catalyst for Negishi coupling reaction (eq. 2)
- Catalyst for Suzuki coupling reaction (eq. 3)
- Catalyst for Buchwald-Hartwig amination reaction (eq. 4)
For small scale and high throughput uses, product is also available as ChemBeads (931063)
Legal Information
This product, its manufacturing or use, is the subject of one or more issued or pending U.S. Patents (and foreign equivalents) owned or controlled by Umicore PMC. The purchase of this product from Umicore PMC through Sigma-Aldrich, its affiliates or their authorized distributors conveys to the buyer a limited, one-time, non-exclusive, non-transferable, non-assignable license. Buyer′s use of this product may infringe patents owned or controlled by third parties. It is the sole responsibility of buyer to ensure that its use of the product does not infringe the patent rights of third parties or exceed the scope of the license granted herein.
For any further information on product please refer to your local Umicore PMC contact at http://www.pmc.umicore.com
Patented, U.S. Pat. No. 7,250,510. Sold under an exclusive license from Total Synthesis Ltd.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Professor Mike Organ, along with Drs. Chris O’Brien and Eric Kantchev, have developed an elegant Pd-NHC catalyst system. The title complex PEPPSI™ stands for Pyridine-Enhanced Precatalyst Preparation Stabilization and Initiation.
Professor Mike Organ and co-workers have developed the PEPPSI™ (Pyridine-Enhanced Precatalyst Preparation Stabilization and Initiation) precatalysts for palladium-catalyzed cross-coupling reactions.
PEPPSI™ Catalysts are novel state-of-the-art catalyst that successfully performs cross-coupling niche reactions.
Professor Mike Organ at York University, along with co-workers Dr. Chris O’Brien and Dr. Eric Kantchev, have developed an palladium N-heterocyclic-carbene (NHC) catalyst system. They reacted PdCl2with a bulky NHC ligand, 2,6-diisopropylphenyllimidazolium chloride (IPr), and an α-donating 3-chloropyridine ligand for stability. The title complex, PEPPSI™, stands for Pyridine-Enhanced Precatalyst Preparation Stabilization and Initiation. Sigma-Aldrich offesr gram-scale quantities of the PEPPSI™ catalyst in collaboration with the Organ research group.
Related Content
Cross-coupling is a common reaction in organic chemistry for the creation of C-C, C-N, and C-O bonds with the aid of a metal catalyst.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![[Pd(IPr#)(cin)Cl]](/deepweb/assets/sigmaaldrich/product/structures/391/578/9bb7eaef-fa70-4f50-8644-2c55cec3925d/640/9bb7eaef-fa70-4f50-8644-2c55cec3925d.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)